Electron Configuration For Hassium: Hassium is a synthetic chemical element that has a symbol Hs. The atomic number of hassium is 108. It is a radioactive and synthetic element. The most stable known isotope of Hassium is 270Hs.
Electron Configuration For Hassium
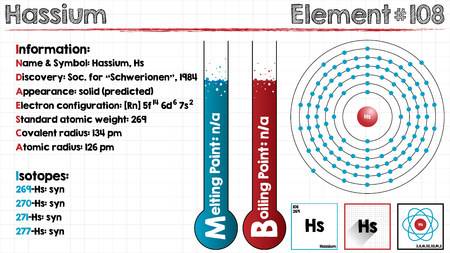
It has a half-life of approx 10 seconds. Almost 100 or more atoms of hassium have been synthesized to date. It is the ad-block transactinide element, in the periodic table of the elements. It is a member of the 7th period which belongs to the group 8 elements. Hassium is, therefore, the sixth member of the 6d series of transition metals. Chemical experiments have confirmed that hassium behaves as the heavier homologue than osmium of group 8.
The chemical features of hassium are characterized partly, but they compare well and fine with the chemistry of the other group 8 elements. In large quantities, it is expected to be a silvery metal which reacts with oxygen readily in the air and forms a volatile tetroxide.
What is the Electron Configuration of Hassium
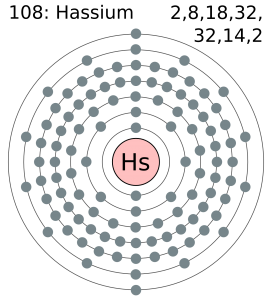
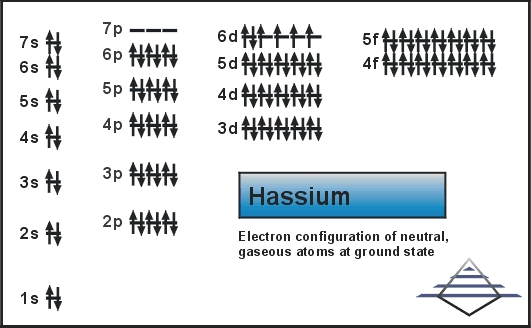
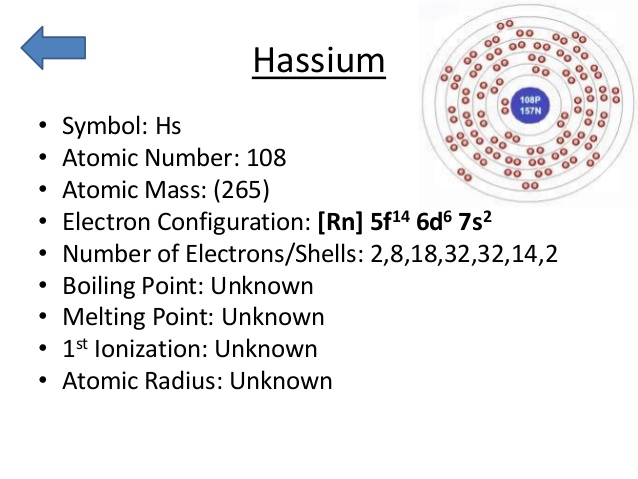
5f14 6d6 7s2 is the electron configuration of the Hassium.
How Many Valence Electrons Does Hassium Have
Hassium has twenty-two valence electrons in its outer shell.
Hassium Number of Valence Electrons
There are twenty-two valence electrons in the outer shell of the Hassium.
Leave a Reply