Learn the Barium electron configuration here and get to explore this chemical element from a close dimension. We are going to cover up the electron configuration and the other basic properties of this element.
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
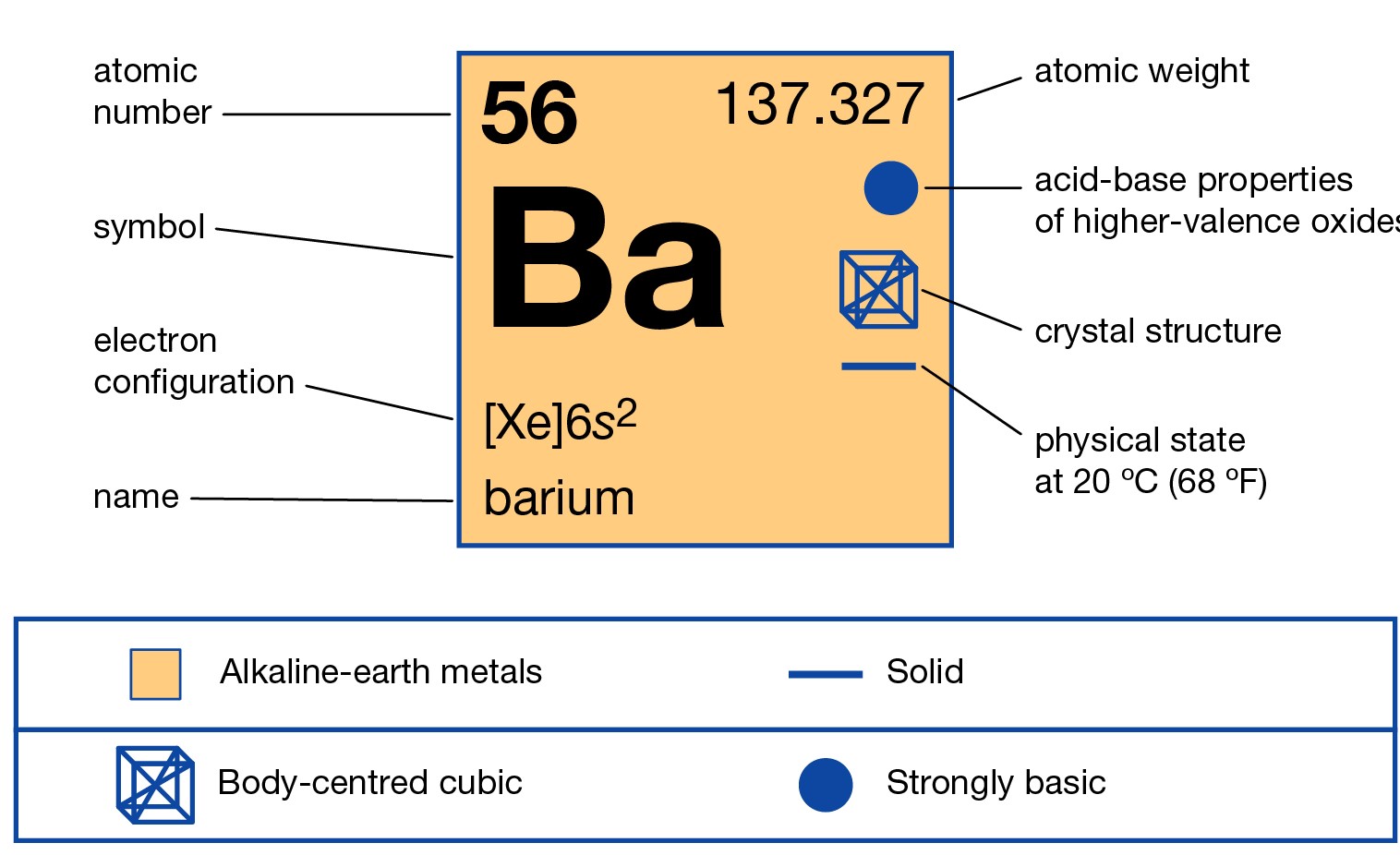
Barium is a chemical element that is highly radioactive in its nature. The element has the atomic number of 56 and the representative symbol of Ba. It belongs to the category of Alkaline earth metals in the periodic table. It has a physical structure as soft silvery and white metal.
Electron Configuration for Barium
There is no free form of the Barium in the earth as it occurs with the other elements. The element occurs in the seawater and also with the baryte mineral. In fact, the majority of the commercial Barium is extracted from the ores of Baryte. The element got its first recognition in the year 1774 so it has quite a significant legacy behind it.
Today Barium is one of the most common chemical elements in science and in its industrial usage. You can easily see its applications in the number of domains. However, the element also has some toxic properties as well which doesn’t make it feasible for human exposure.
Barium Electron Configuration
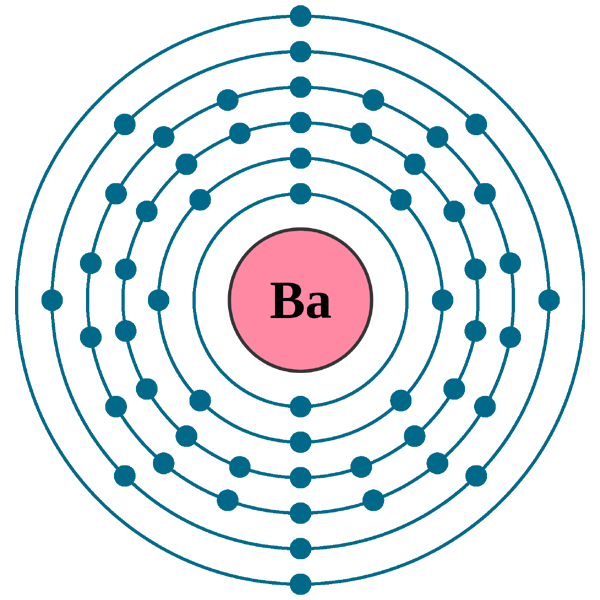
The electron configuration of Barium is [Xe] 6s2 which is its abbreviated form. We urge you to learn the electron configuration formula for the chemical elements. You can also remember this electron configuration of Barium for future reference of yourself. These are some of the significant aspects of the electron configuration for any element.
- The valency of the chemical element is the direct consequence of the electron configuration for such an element.
- Electron configuration helps in breaking out the whole element and finding its more appropriate usages.
- The chemical reaction is the other thing that is also determined with the help of electron configuration.
How many valence electrons does Barium have?
Barium is a significant chemical element and stands out uniquely just like any other element. The element is significantly useful in the commercial industry. For instance, Barium as a metal is useful to be used in the integration of aluminum.
It works by removing the harmful gases from the various tubes such as the tv tubes and other electronic appliances. In fact, Barium also works as the portent source of removing the noble games from unwanted places. The chemical element is useful in its other such purposes however it’s not safe for human exposure. We believe that all these details of this element would make it easier to understand in a better way.
Leave a Reply