Get to understand the Oganesson electron configuration here in our article and build a solid knowledge of the chemical element. Here in the article, we are providing useful information on the Oganesson chemical properties and its electron configuration.
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
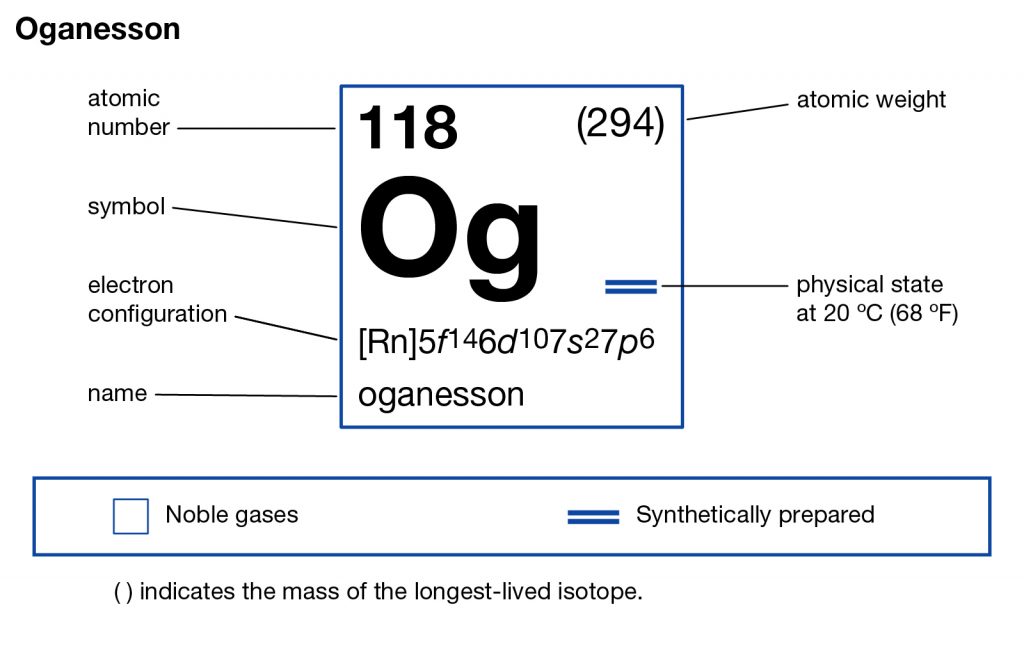
In the Chemistry branch of science, the Oganesson is the synthetic chemical element that has the atomic number 118 and the symbol of Og. The element was discovered by the Russian and American scientists together in an experiment during 2002. It’s, therefore, one of the most recently developed chemical elements.
Electron Configuration for Og
Oganesson is also one of those chemical elements that were named after the name of people who were actually alive at the time of naming. This is some other unique kind of fact that makes this chemical element special in itself. Furthermore, Oganesson has its atomic mass number 294 along with its other chemical properties that you can check in the periodic table.
Oganesson is also one such chemical element that comes with the highest atomic number for a chemical element. The chemical element is highly radioactive in its nature and therefore has no stable isotope as of now. You can find the Oganesson in the p block of the periodic table and also the last element from period 7.
Oganesson Electron Configuration
Oganesson electron configuration is one of the highly significant chemical properties of the element that you should study. The chemical element has the electron configuration as Rn]7s27p65f146d10 for the information of our readers. The electron configuration equation here represents the distribution of its electrons to the atomic orbitals.
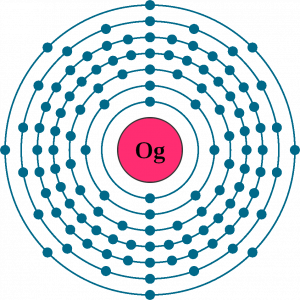
The electron configuration is the naturally occurring or integral chemical property of the element. In chemistry, all the chemical elements have their own respective chemical properties that are useful for a number of reasons. Here is the significance of the Oganesson electron configuration.
- The electron configuration is crucial, in order to break out the whole chemical element and finding its true nature.
- The electron configuration also helps in determining the valency of the element.
- Scientists can explore the elements in the best possible manner and find their more suitable usages.
So, due to all these factors, the electron configuration of Oganesson is highly significant in itself. You, therefore, need to have a clear understanding of the electron configuration for Oganesson.
How many valence electrons does Oganesson have?
Well, as we know that Oganesson is a purely synthetic chemical element that comes with huge radioactive chemical properties. The chemical element is therefore highly toxic and lethal in its nature. It’s therefore not available for any of the practical purposes as of now. However, in its research phase, the element is being extremely useful in the domain of nuclear energy.
Scientists are still trying to harness the extreme power of the element so as to find its safe usage. It’s believed that the Oganesson will be highly useful in the domain of nuclear energy. We hope that the article will come very useful in explaining the Oganesson electron configuration to all the readers.
Leave a Reply