Today we shall discuss the valency of another important element, i.e., Phosphorus Valence Electrons. As a chemistry student, you need to remember the valency of all the important elements in the periodic table. Phosphorous is a waxy red or white coloured element. Since it is a highly reactive element, it is never found free in nature and is mostly found in the oxidized state in phosphate rocks.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
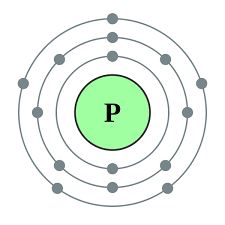
Since phosphorus has 5 electrons in its outermost shell, it has the valency of 5.
Why is the Valency of Phosphorus 3 and 5
Phosphorous shows valencies of both 3 and 5. It depends upon the electronic configuration of the atom. In case there are 3 unpaired electrons in its three p orbitals, it has a valency of 3. For example, in case of PCl3.
When Phosphorus has a valency of 5, like in PCl5 , one of 3s electron gets promoted to 3d orbital.
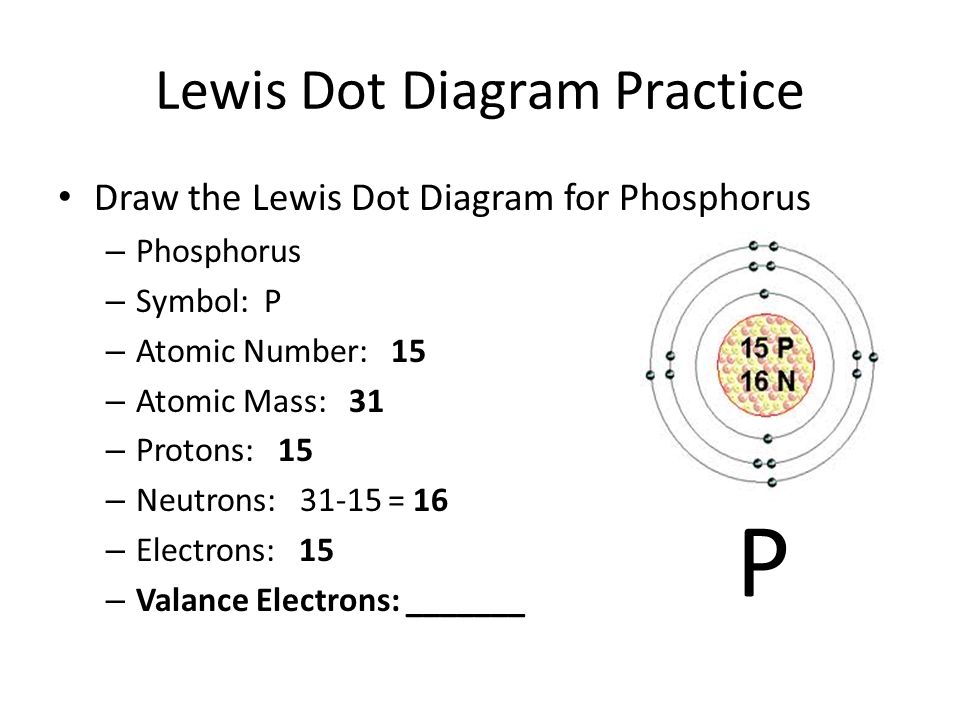
Phosphorus Valence Electrons Dot Diagram
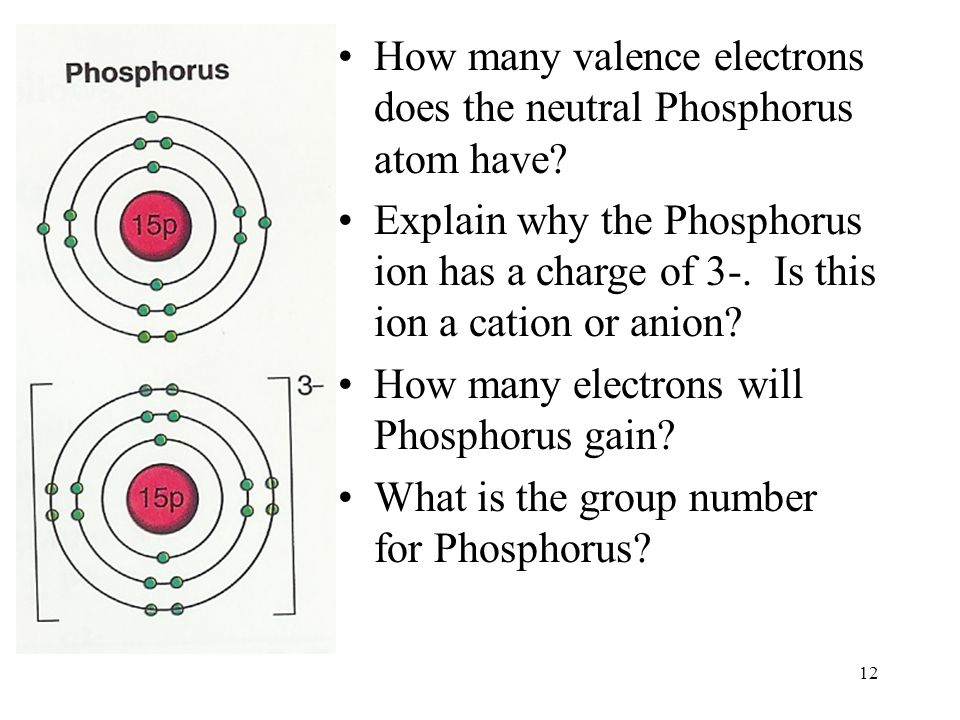
The atomic number of a Phosphorous atom is 15. The 1s shell contains 2 electrons, the 2s shell contains 2 electrons and the 2p shell contains 6 electrons. So the outermost shell contains 5 valence electrons. So these 5 electrons can be distributed in the following manner: 2 can go into the 3s subshell, and the remaining 3 electrons can go into the 3p subshell.
How many valence electrons does Phosphorous have?
Thus, valence electronic configuration of neutral phosphorus atoms is 1s22s22p63s23p3.
What Is The Valence Electron Configuration For Phosphorus
The valence electronic configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3
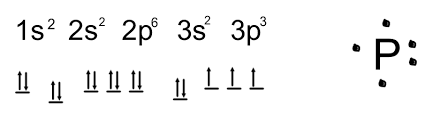
Leave a Reply