d fhe valency of an element is the potential or the tendency of an element to combine with the other atoms. So today we shall discuss on the topic What is Valency With Examples. It is especially the measure of how many Hydrogen atoms can an element can displace or combine with. All the elements in the same group in the periodic table have the same valency.
What is Valency With Examples
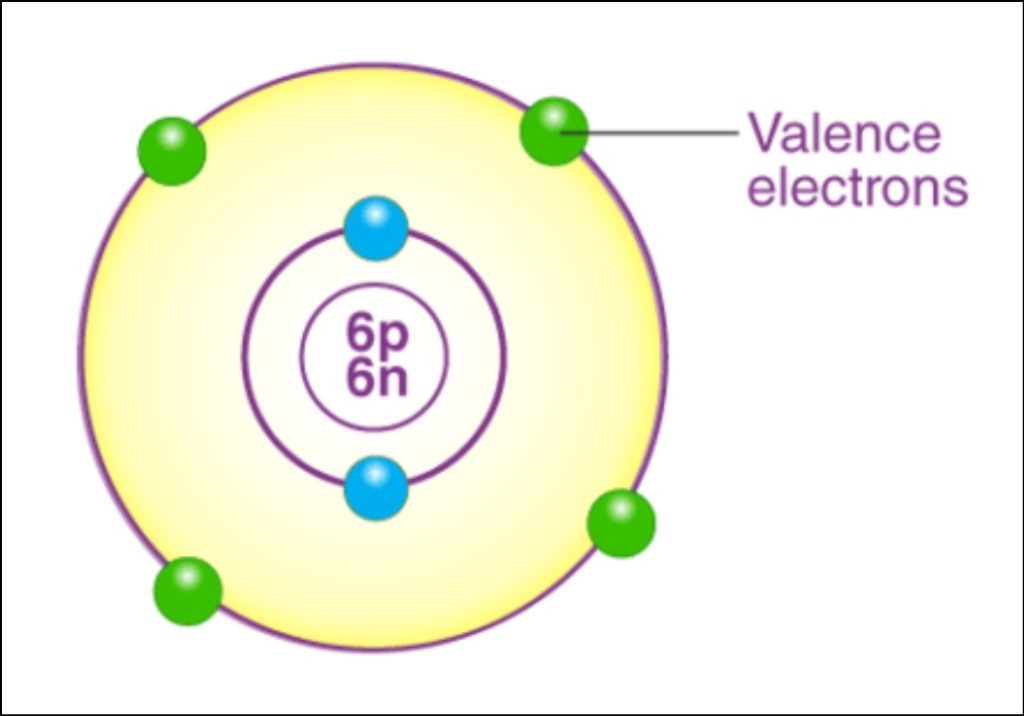
Valency, in chemistry, refers to the combining capacity of an element or an ion. It indicates the number of chemical bonds an atom of that element can form to attain a stable configuration. The concept of valency is crucial in understanding chemical reactions and the formation of compounds. Valency is determined by the number of electrons an atom can gain, lose, or share to achieve a stable electron configuration.
For example, let’s consider the element hydrogen (H). Hydrogen has one valence electron in its outermost shell. To attain stability, it can either gain one electron to form H-, lose that one electron to form H+, or share one electron with another atom to form a covalent bond, as seen in H2. Hence, hydrogen’s valency is 1.
Another example is oxygen (O), which has six valence electrons. To achieve a stable configuration, oxygen can gain two electrons to form O2-, lose all six electrons to form O6+, or share two electrons with other atoms in a covalent bond, such as in O2 molecules. Therefore, oxygen’s valency is 2.
In summary, valency is a crucial concept in chemistry that helps us understand how elements bond to form compounds, and it is determined by the number of electrons an atom can gain, lose, or share to achieve stability. Learn How To Find Valency of Elements
How Valency is Calculated For an Element
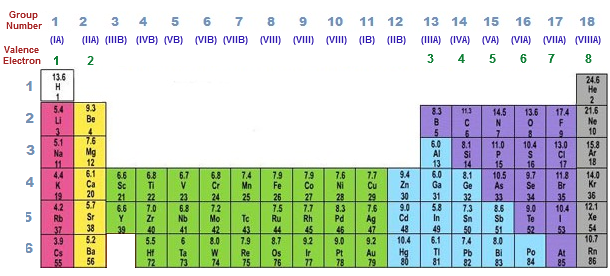
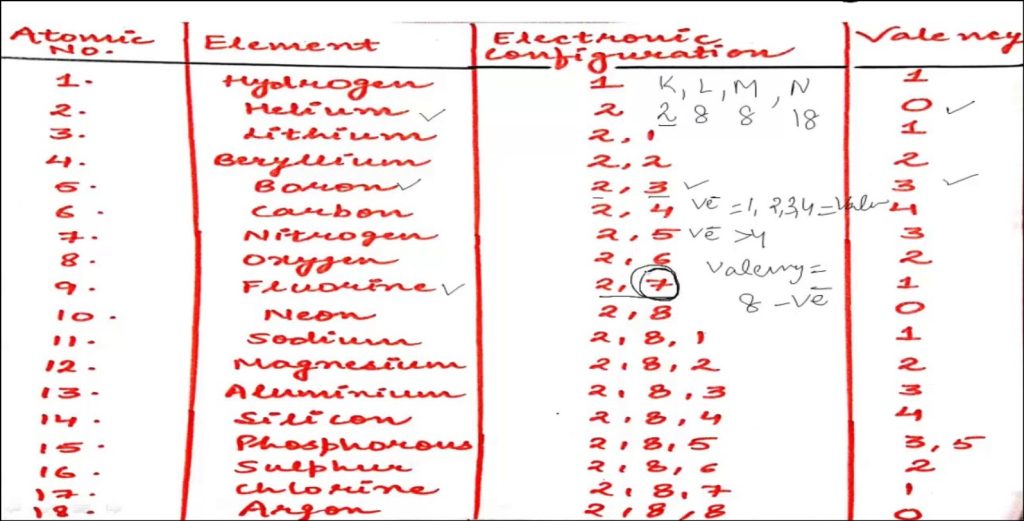
Valency is determined by the electron configuration of an element’s outermost shell, also known as the valence shell. The valence shell contains the electrons that are involved in chemical bonding. To calculate the valency of an element, we need to look at its position in the periodic table.
For main-group elements (those in groups 1, 2, 13-18), the valency is typically equal to the group number. And for instance, elements in group 1 (e.g., lithium, sodium) have a valency of 1, while elements in group 2 (e.g., magnesium, calcium) have a valency of 2.
For transition metals (those in groups 3-12), determining valency is slightly more complex. The valency of transition metals can vary depending on the specific compound they form. The valency of transition metals is often indicated using Roman numerals to represent different oxidation states. For example, Iron (Fe) can have a valency of 2 (Fe2+) or 3 (Fe3+), depending on the compound.
To calculate What is Valency With Examples of an element, it’s essential to consider its electronic configuration and its position in the periodic table, keeping in mind the exceptions for transition metals.
How Many Types of Valency Are There
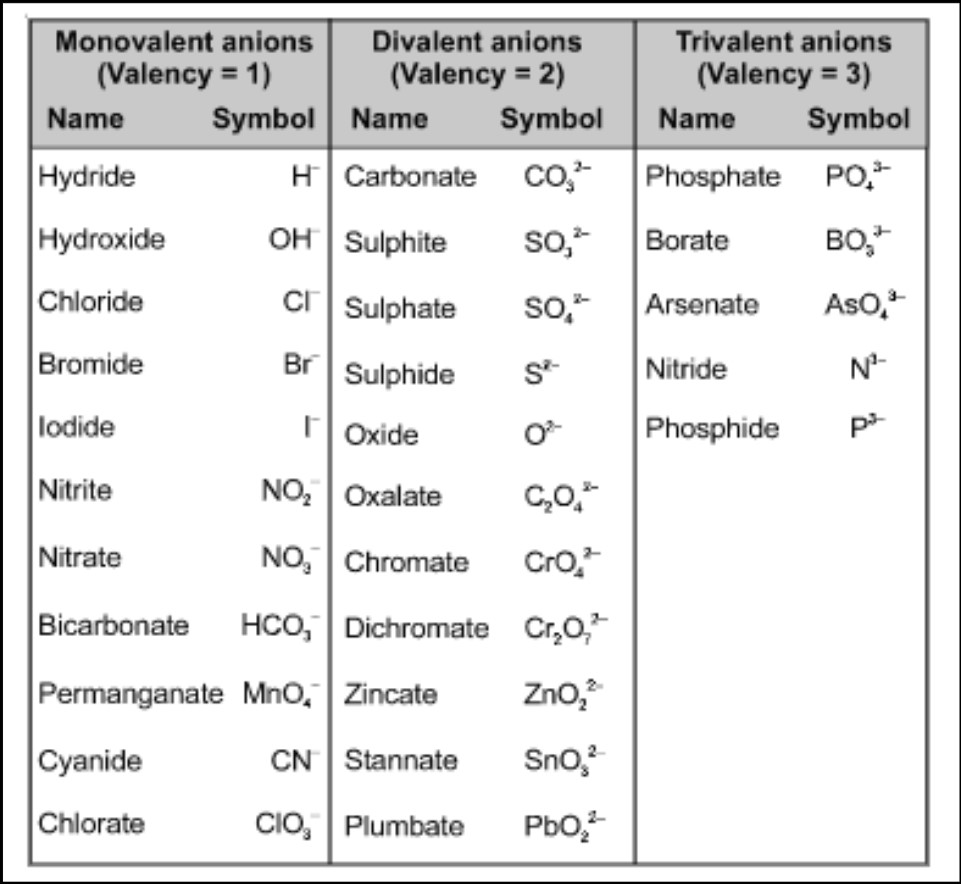
There are two primary types of valency: positive valency and negative valency.
- Positive Valency. This type of valency refers to the ability of an atom to lose electrons and form positive ions (cations). Elements with positive valency are usually found on the left side of the periodic table. For example, group 1 elements like sodium (Na) have a positive valency of 1, meaning they can lose one electron to form Na+.
- Negative Valency. Negative valency, on the other hand, pertains to the ability of an atom to gain electrons and form negative ions (anions). Elements with negative valency are typically located on the right side of the periodic table. For example, group 17 elements like chlorine (Cl) have a negative valency of 1, meaning they can gain one electron to form Cl-.
It’s important to note that the valency of an element can vary depending on the chemical reaction it undergoes. For example, oxygen can have a negative valency of 2 when forming compounds like O2-, but it can also exhibit a valency of -1 in compounds like H2O2 (hydrogen peroxide), where it gains an extra electron.
How Do We Calculate Valency
The What is Valency With Examples of an element can be calculated using the following steps:
- Determine the Electron Configuration. Find the electron configuration of the element, specifically focusing on the outermost shell (valence shell).
- Identify the Number of Valence Electrons. Count the number of valence electrons in the outermost shell. This number corresponds to the valency of the element.
- Determine the Type of Valency. Based on the number of valence electrons, determine whether the element exhibits positive valency (tending to lose electrons) or negative valency (tending to gain electrons).
- Consider Exceptional Cases. Keep in mind that transition metals may have variable valencies due to the involvement of inner electron shells in bonding. In such cases, Roman numerals are used to represent the different valency options.
- Observe the Periodic Table. For main-group elements, the group number generally represents the valency (except for hydrogen). Elements in the same group tend to have similar valencies.
By following these steps and understanding the periodic trends, you can calculate the valency of most elements, helping you predict their chemical behavior and interactions with other elements to form compounds.
Leave a Reply