Sulphur is an important compound from the point of view of chemistry. So we shall talk about Valency Of Sulphur today. Sulphur is a non-metallic element yellowish in colour which is abundantly found in the earth.
What is the Valency of Sulphur Why
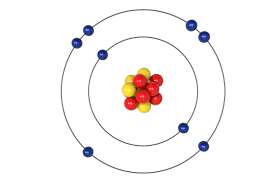
Sulphur has the atomic number of 16. Its electronic configuration is 2,8,6. The valency of S depends upon its oxidation state and with which element it reacts.
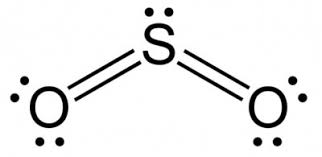
For example, the valency of S in SO2 is +4 and in SO3 is +6.
How Many Valence Electrons Are Atom Of Sulfur
It depends upon in which state it is found and its reaction with another element. The atomic number of sulphur is 16. Hence it has 2 electrons in its first shell, 8 electrons in its second shell. So the balance number of electrons which can come in its third shell is 6. Hence the valency of S is 6.
What Is The Valency Of Sulphur In SO2
The valency of S in SO2 is 4. It is because both the oxygen atoms have a valency of 4 and the S has a valency of 6. So together they stabilize each other and share their electrons to form an octet.

Leave a Reply