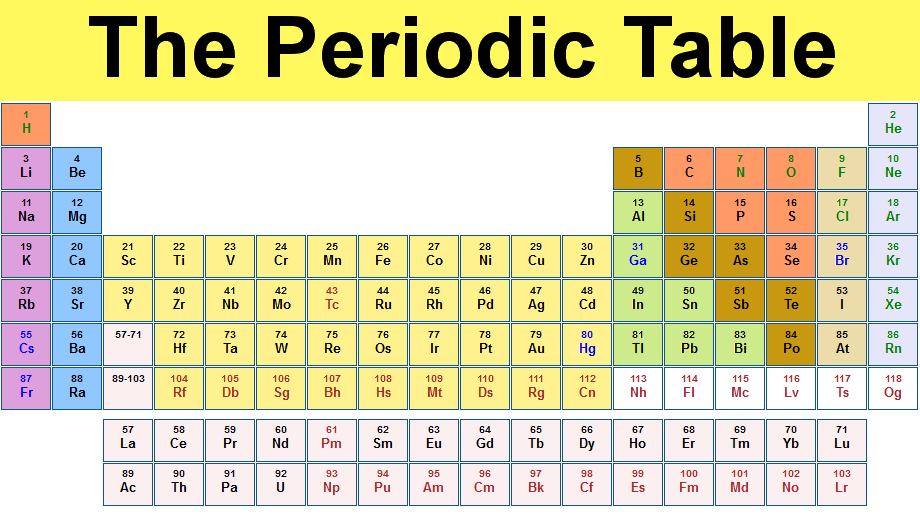
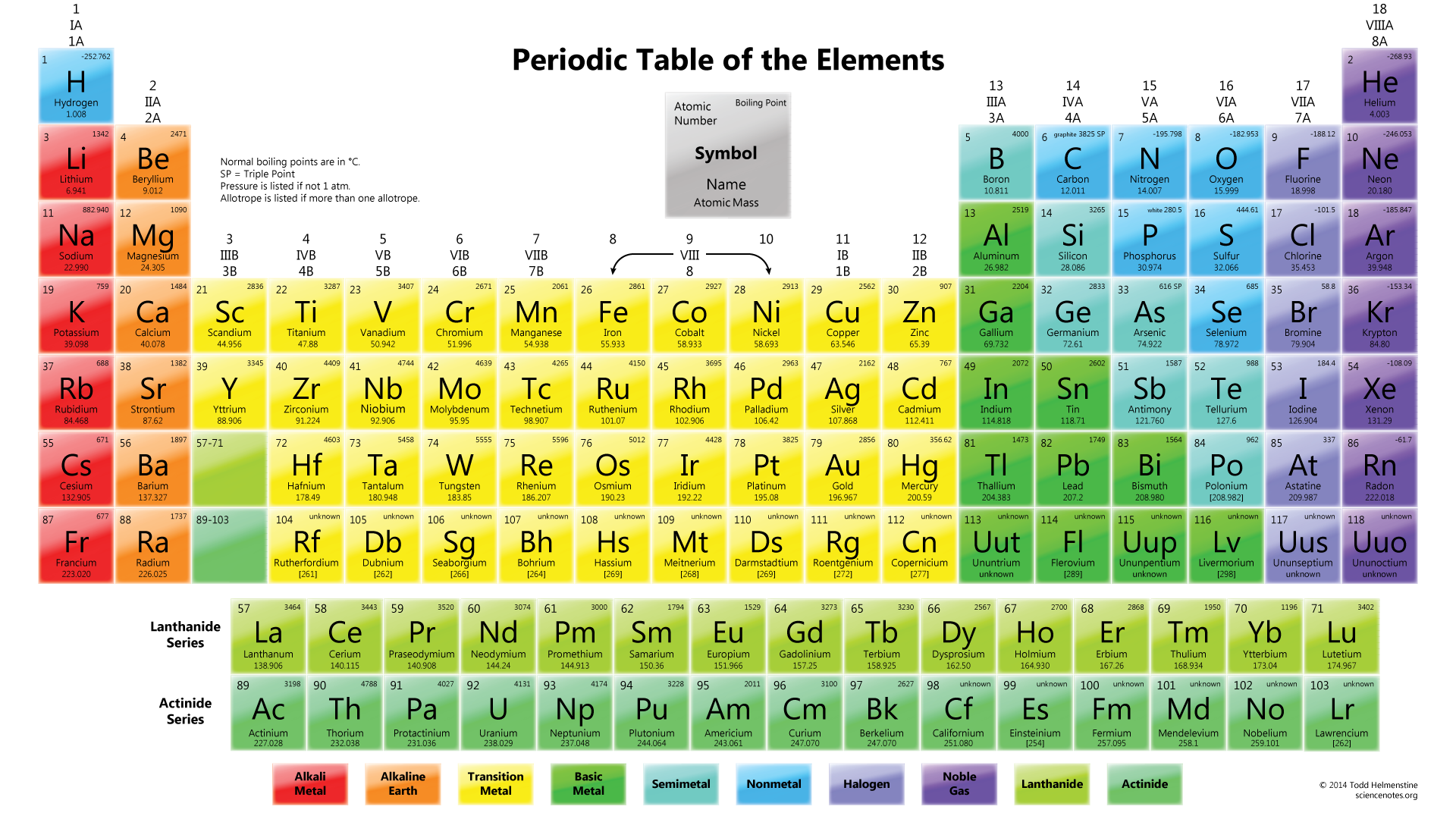
Periodic Table is the table which arranges all elements in a particular order. That order is the increasing order of element’s atomic mass, recurring chemical properties and configuration of electron. It considered as the most crucial tool in the history of chemistry. It helps to describe the atomic property of each chemical element. Elements that have same chemical properties arranged in columns in the periodic table. It helps to describe the atomic structure of all elements. For example just by looking at the periodic table we can find out that how many electrons the element possess and what is the weight of those electron.
Every element have its own different set of such data as no two elements are the same. Therefore, if someone is uncertain what element they have, they can look at the atomic structure of that element and compare it to the data in the periodic table and finally identify the element by matching to the element in table with the similar data. Elements in the periodic table are classified in different families and periods. The elements in each period and family may or may not have similar or dissimilar characteristics. The table thus is considered as reference to which element behave the same or which may have atomic structures or same weight.
What Is a Period in the Periodic Table
In the Periodic Table, a period refers to a horizontal row of elements that share similar electronic configurations and exhibit recurring trends in their properties. The elements within a period are arranged in order of increasing atomic number, meaning that as one moves from left to right across a period, the atomic number increases by one.
Each period corresponds to a new electron shell being added to the atoms of the elements in that row, leading to an increase in the number of energy levels for the electrons. The period number is denoted at the top of each row, ranging from 1 to 7 in the modern Periodic Table.
Each period showcases specific trends in atomic size, electronegativity, ionization energy, and electron affinity as one moves across it. This variation in properties is attributed to the change in electron configuration, which influences how the elements interact with other substances.
Elements belonging to the same period do not necessarily have the same chemical properties, but they do share certain similarities due to their electronic structure. Understanding the periodicity of elements in the table is fundamental to the field of chemistry and helps predict their behavior and reactions. Learn How & Why Is The Periodic Table Organized here.
How Many Periods are in the Periodic Table
The Periodic Table consists of seven periods, which are the horizontal rows of elements running from left to right. Each period represents a different electron shell or energy level, with the first period containing only two elements, hydrogen and helium. As we move from left to right across the table, additional elements are added to each period, reflecting the sequential filling of electron orbitals in atoms. The seventh period, the one with the highest principal quantum number, is the largest and currently includes 32 elements.
It is important to note that elements in the same period do not possess identical chemical properties, as these are primarily determined by the elements’ electron configuration and the arrangement of valence electrons. The period number on the periodic table indicates the highest energy level occupied by electrons in the elements of that row. For example, elements in the third period have electrons in energy levels up to the third shell, and so on.
What Do The Numbers on a Periodic Table Mean
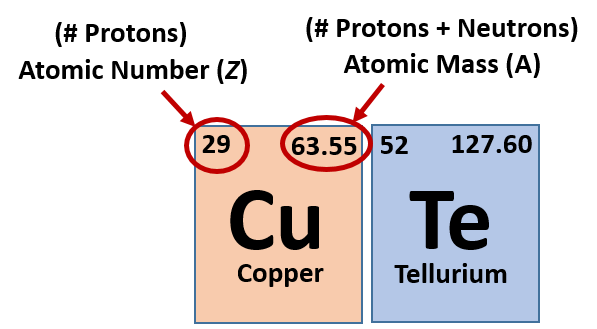
The numbers displayed on a Periodic Table serve a crucial purpose in identifying and organizing elements. Two types of numbers are commonly found on the table: the atomic number and the atomic mass.
The atomic number represents the number of protons in an atom’s nucleus, which is a unique identifier for each element. It defines an element’s chemical properties and determines its place in the Periodic Table. The elements arranged in ascending order of atomic number, from left to right, in periods, and in groups with similar properties.
The atomic mass, on the other hand, represents the average mass of all naturally occurring isotopes of an element, taking into account their relative abundance. It usually given in atomic mass units (amu) and can found beneath the element’s symbol in each cell of the table.
Additionally, the Periodic Table organized into groups (columns) and periods (rows). Elements within the same group have similar chemical properties as they have the same number of valence electrons. The group number reflects the number of valence electrons in the outermost shell of elements in that group. Conversely, the period number corresponds to the highest energy level occupied by electrons in the elements of that row.
Who Made the Periodic Table
The Periodic Table is one of the most significant achievements in the history of chemistry, and its creation can attributed to Dmitri Mendeleev, a Russian chemist. In 1869, Mendeleev published the first widely recognized version of the Periodic Table, though there were other attempts at organizing the elements before him. Mendeleev’s approach was revolutionary because he organized the elements based on their atomic weights and observed repeating patterns in their chemical properties.
Mendeleev arranged the elements in a tabular format, leaving gaps for elements that yet to discovered. He made several key predictions, such as the existence and properties of elements like gallium and germanium, which subsequently found and validated his predictions. His work provided a solid foundation for the development of modern periodic law and the table we use today.
Over time, the Periodic Table has undergone refinements and modifications to accommodate newly discovered elements and align with the current understanding of atomic structure. Nonetheless, Mendeleev’s original contributions and insight into the periodicity of elements remain pivotal to the study of chemistry.
What is the Periodic Table of the Elements
The Periodic Table of the Elements a systematic arrangement of chemical elements, where these elements organized based on their atomic number, electron configuration, and recurring chemical properties. It provides a comprehensive and concise way to represent the entire set of known elements, their properties, and relationships. The layout of the Periodic Table consists of periods (rows) and groups (columns).
Each element represented by its chemical symbol, usually a one or two-letter abbreviation, and placed in a specific cell on the table. The cells organized in such a way that elements with similar properties grouped together. Elements in the same group generally have the same number of valence electrons, which plays a significant role in determining their chemical behavior and reactivity.
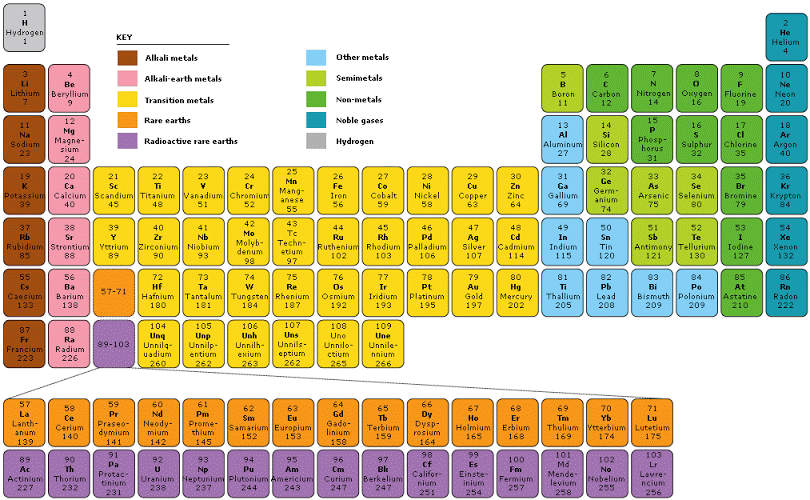
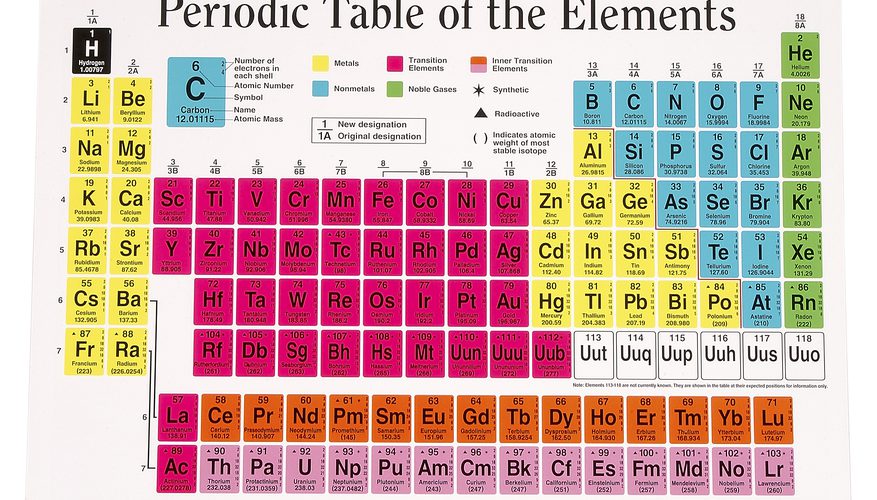
The Periodic Table divided into several blocks, including the s-block, p-block, d-block, and f-block, based on the type of subshell that receives the last electron during the filling of electron orbitals. The s-block and p-block elements are typically the most familiar and make up the majority of elements encountered in everyday life.
This versatile tool is invaluable to chemists, researchers, and students as it provides a visual representation of the relationships and trends among elements, allowing for easy comparison and prediction of their properties and reactions.
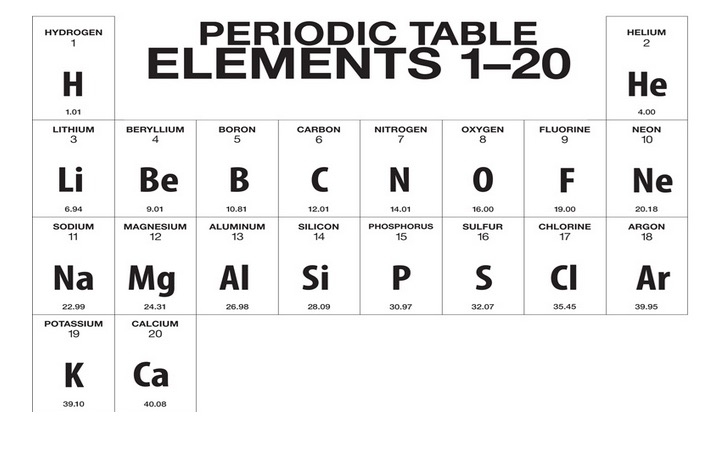
What is the First 20 Elements of the Periodic Table
The first 20 elements of the Periodic Table include a diverse range of elements that exhibit varying chemical and physical properties.
Listed below are these elements along with their atomic numbers and chemical symbols:
- Hydrogen (H) – Atomic Number 1
- Helium (He) – Atomic Number 2
- Lithium (Li) – Atomic Number 3
- Beryllium (Be) – Atomic Number 4
- Boron (B) – Atomic Number 5
- Carbon (C) – Atomic Number 6
- Nitrogen (N) – Atomic Number 7
- Oxygen (O) – Atomic Number 8
- Fluorine (F) – Atomic Number 9
- Neon (Ne) – Atomic Number 10
- Sodium (Na) – Atomic Number 11
- Magnesium (Mg) – Atomic Number 12
- Aluminum (Al) – Atomic Number 13
- Silicon (Si) – Atomic Number 14
- Phosphorus (P) – Atomic Number 15
- Sulfur (S) – Atomic Number 16
- Chlorine (Cl) – Atomic Number 17
- Argon (Ar) – Atomic Number 18
- Potassium (K) – Atomic Number 19
- Calcium (Ca) – Atomic Number 20
These elements encompass a wide range of properties. Hydrogen and helium are light, gaseous elements found in the first period. Moving through the second and third periods, we encounter metals like lithium, beryllium, and sodium, as well as nonmetals like boron and carbon. In the fourth period, elements like phosphorus and sulfur are important components of organic compounds, while chlorine is a potent halogen. The fifth period includes noble gases like argon, which are unreactive. Elements such as potassium and calcium in the sixth period are crucial for biological processes.
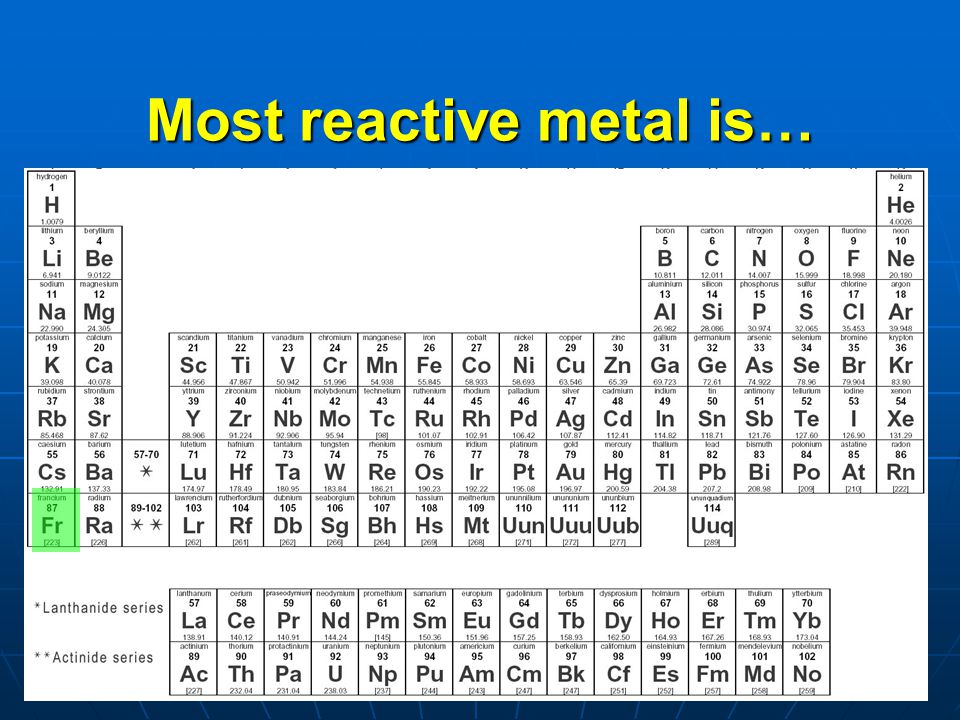
What is the Most Reactive Metal on the Periodic Table
The title of the most reactive metal on the Periodic Table attributed to francium (Fr). Francium is an extremely rare and radioactive element with the atomic number 87. It belongs to the alkali metal group, which known for its high reactivity due to the presence of a single valence electron in their outermost electron shell.
Because of its high reactivity, francium never found in nature in significant quantities. It a product of the radioactive decay of other elements and typically synthesized in laboratories for scientific research. Its extreme radioactivity and scarcity make it a challenging element to study, and most of what we know about it comes from theoretical calculations and predictions based on its position in the Periodic Table.
Due to its radioactivity, francium poses significant health hazards and not used for any practical applications. Its short half-life and limited availability also contribute to the difficulties in studying its chemical properties. Nevertheless, francium’s reactivity reflects the trend of alkali metals becoming increasingly reactive as you move down the group in the Periodic Table. Elements like cesium and rubidium, which come before francium in the same group, are also highly reactive, but francium surpasses them in terms of reactivity.