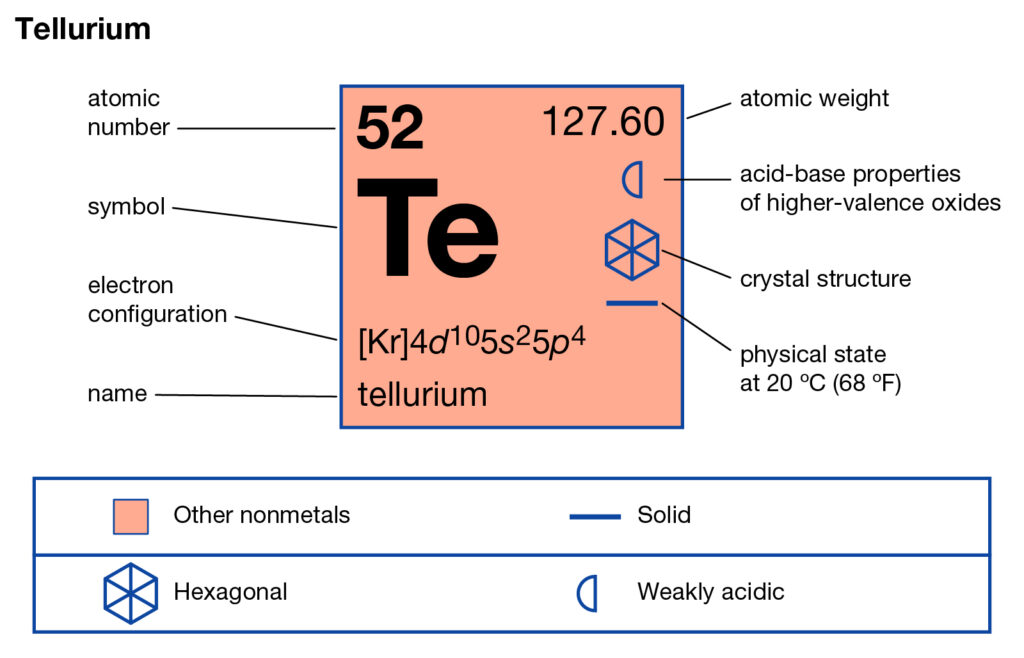
Read about the Tellurium valence electrons here and explore the element in depth. You can also read about the other properties of Tellurium in the article. Tellurium is the chemical element in the chemistry branch of science. The element has the atomic symbol Te and the atomic number 52.
How many valence electrons does Tellurium have?
In appearance, Tellurium looks like a brittle silvery and white element. It belongs to the family of metalloids hence it has similar properties. Tellurium has chemical properties similar to Sulfur and Selenium. It has several types of occurring forms in nature.
The major Tellurium’s occurrence form includes the refining process of blister copper. Some form of tellurium is also available in the elemental crystals. Tellurium was first discovered in the year of 1782 in the mines of gold, however, it was not enough source of its availability. The element is significance available as the byproduct of gold and lead.
So, Tellurium basically derives itself from copper and lead and is also useful with the same components. The major use of Tellurium is in integration with copper and steel.
Tellurium makes steel, copper more machinable for their effective usage. So, hence metallurgy domain is the largest consumer of tellurium.
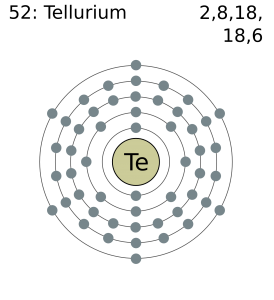
Tellurium Valence Electrons Dot Diagram
The dot diagram helps in the better representation of Te valence electrons. It shows the numbers of tellurium valence electrons of atoms.
In a similar way, the diagram also provides the presentation of tellurium chemical bonding. So, the dot diagram provides the best picture of Te valence electrons analysis.
Valency of Tellurium
Well, Tellurium has 6 valence electrons in the outermost energy shell. The valency of tellurium is the combining capacity of elements.
It determines that how the atoms of tellurium will react with other elements. You can also check out the valency of tellurium in the periodic table.