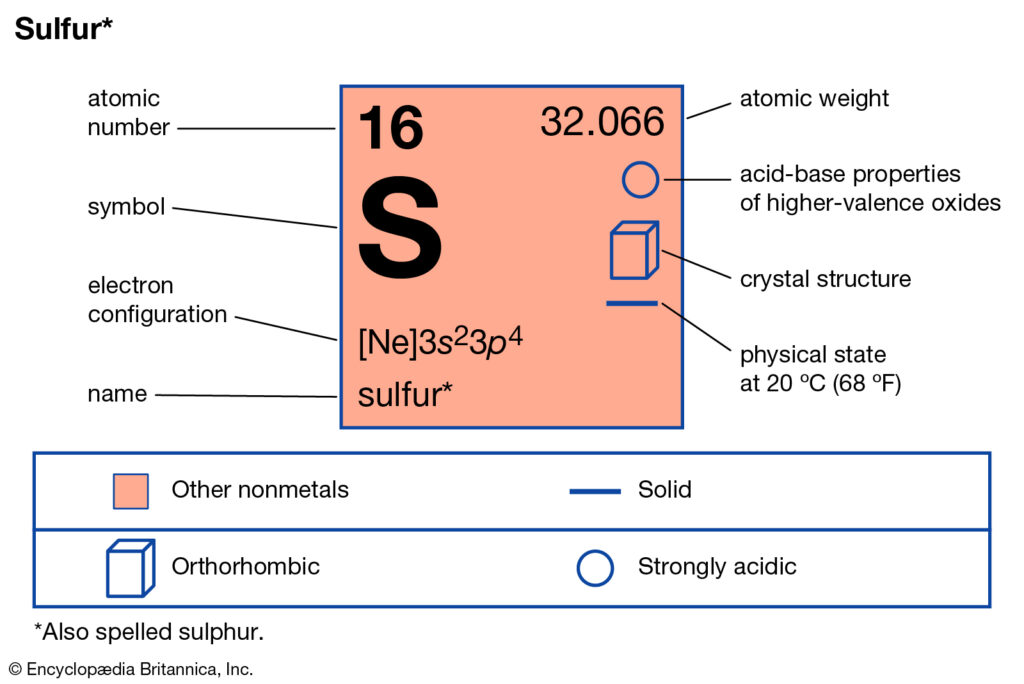
Get to know about the sulfur valence electrons and enhance your science knowledge. In the article, we shall talk about the electron valence of Sulfur. Sulfur is basically a chemical element in the chemistry branch of science. The chemical is known for its abundance availability across the earth.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Sulfur is the world’s fifth-most abundantly found chemical element on earth. The element has applications in chemical processing, fertilizers, pesticides, etc. The chemical element has a bright yellow and solid composition and it is adjustable in room temperature. Other than this, sulfur has enormous usages across the pharmaceutical industry as well.
Sulfur Valence Electrons Dot Diagram
Well, with the dot diagram you can witness the representation of electrons for the atom. We also know the diagram as the lewis electron dot diagram.
It’s an ideal diagram to understand the electron valence in the outermost of the atom. In the dot diagram pair of dots represents the single bond. In a similar manner double pair of dots shows the double bond. We advise you to go through the dot diagram of sulfur for better insight.
Valency of Sulfur – S
The valency of sulfur is actually very subjective since it varies. In a few situations, sulfur may have the valency of two while in other cases it may be six. Sulfur basically holds six electrons in its outer shell. In the general scenario, it’s easier for the sulfur to gain 2 electrons rather than losing six.
How many Valence Electrons does Sulfur have?
This is the reason that why sulfur may have either two or six electrons in its valency. The valency of sulfur is subjective since it is up to the reaction of sulfur in the given scenario. You may read more about the variance of sulfur on the internet. It would provide you with decent exposure to sulfur valency.