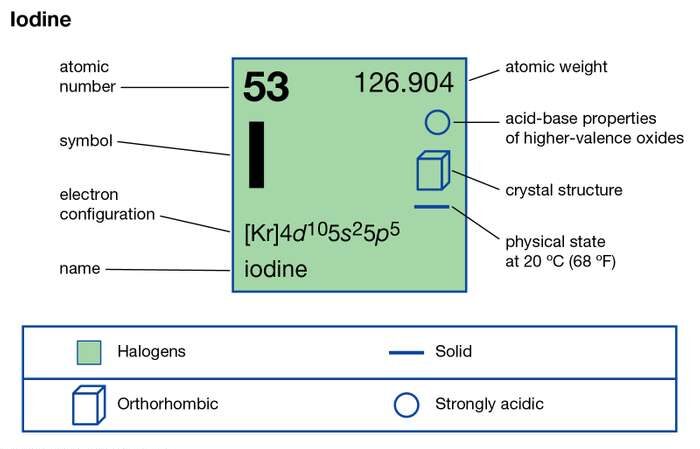
Read about the Iodine valence electrons here, so as to enhance your chemical elements knowledge. We provide the systematic study of chemical elements to the readers of chemistry. Iodine is recognized as the chemical element in the domain of chemistry. It has the atomic number of 53 and the representative symbol as I.
How many valence electrons does Iodine have?
It has a lustrous solid nonmetallic purple and black color form. Iodine although appears solid, but it easily sublimes with a very gentle heat. Iodine is the heaviest chemical element in the category of mineral nutrients. It occurs or takes place in the several oxidation or anions periodate states.
Iodine is prominently known as the thyroid nutrients. Hence it has the major use of providing the proper nutrition as the synthesis of the thyroid. Lack of Iodine may result in the formation of severe diseases relating to the thyroid. This is the reason why the Thyroid is an integral ingredient of food supplements.
So, the major consumption of iodine goes to the food supplement industry. Further stats show that each year the lack of iodine affects nearly two billion people. Today, Japan and Chile are the major producers of Iodine in the world. They export and meet out the significant demand of this highly nutrient chemical element.
Iodine Valence Electrons Dot Diagram
Understand the exact numbers of Iodine valence electrons with the Lewis dot diagram. The diagram further represents the chemical binding of Iodine valence electrons.
In this diagram, we basically draw the numbers of dots around the symbol of Iodine. The numbers of dots represent the numbers of valence electrons of atoms.
Valency of Iodine
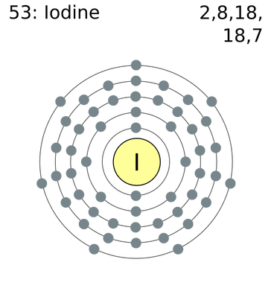
The valency of Iodine remains -1 for most of its states. The chemical element has 7 electrons in the outer shell of energy. Hence Iodine gains 1 electron to attain the state of stability. Furthermore, Iodine is a member of group 17 in the periodic table