Let’s explore the Argon valence electrons and simplify this chemical element. We shall discuss the electron valency and other details of Argon in the article. Argon is a chemical element that we study in the chemistry branch of science. The chemical element is denoted by the symbol Ar.
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
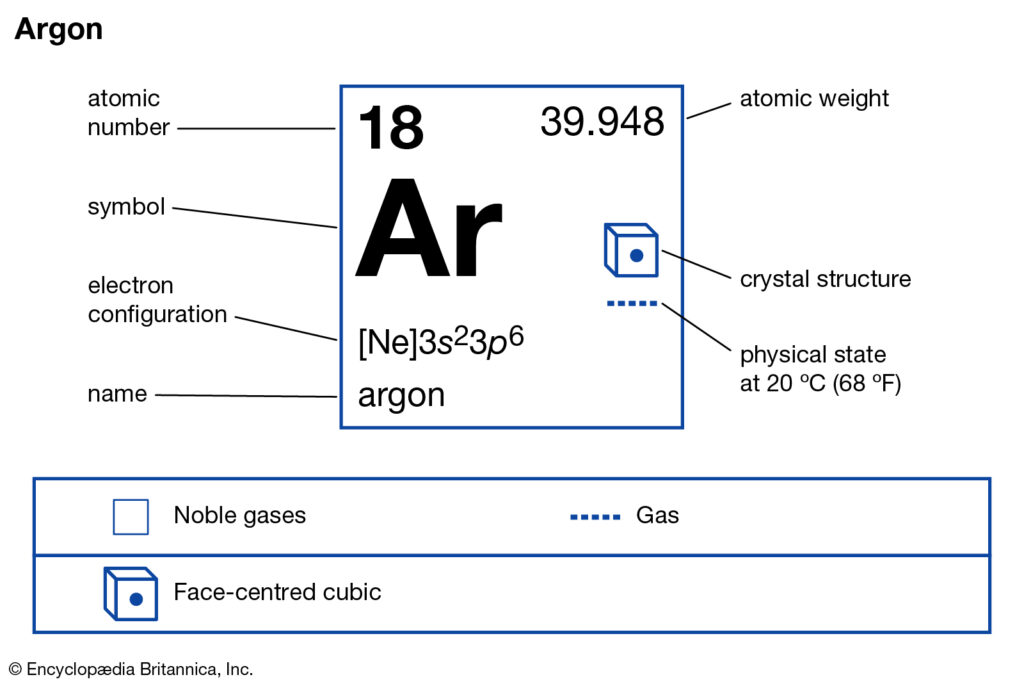
How many Valence Electrons does Argon have?
Argon is originally a noble gas element in the atmosphere of the earth. It’s one of the most abundant noble gas forms in the atmosphere even more than water vapor. The application of Argon varies from industrial use to the substitution of nitrogen. Argon is the cheapest and the best possible alternative of nitrogen.
Argon gas is deadly in the scenarios where it holds more place than oxygen availability in the environment. It is also useful to increase the shelf life of various products due to the same reason. In a similar manner Argon gas is highly useful in the various domains. The gas can be used or abused as per the desired outcome.
Argon Valence Electrons Dot Diagram
Dot diagram is all about representing the electrons in the outer shell of the atom for the element. The dot diagram basically visualizes the valence electrons and molecules. Position of Argon also given on the periodic table.
It figures out whether the valence electrons of atoms and molecules exist in single pair or in bonds. The single dot pair represents the single bond. Double pair of dots represents the double bond. The technique is useful in the proper analysis of electron valence and molecules.
Valency of Argon
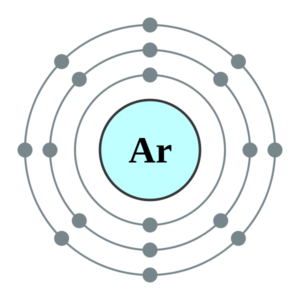
Well, Argon has an absolute zero valency. Argon holds 18 electrons in its outer shell with the electron configuration of 2,8,8. So, this is why Argon has no need to either gain or lose the electrons. The eight electrons fill the valence shell to bring about the 0 valencies of Argon