Silicon Electron Configuration: Silicon is the chemical element with atomic number 14. This is a brittle and hard Crystalline solid which has the metallic lustre of blue and grey. It is a semiconductor and tetravalent metalloid. The symbol of silicon is “Si” and belongs to the 14th group of periodic table and it is above Carbon. Electron configuration is the distribution of electrons in the shells or orbits of the atoms and molecules.
Silicon Electron Configuration
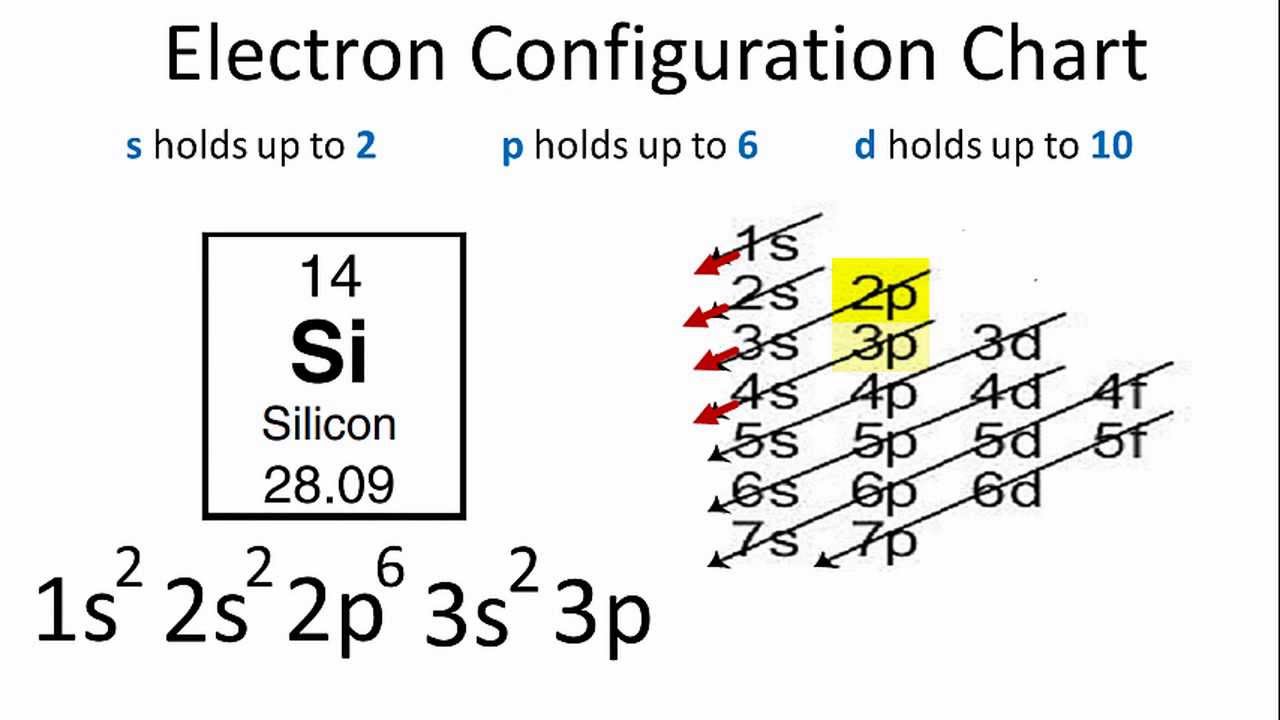
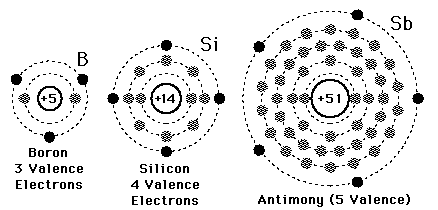
Electrons of silicon per shell are 2, 8, 4. The electronic configuration for silicon is:
1s22s22p63s23p2.
Electron Configuration For Silicon ion
The electron configuration for silicon ion can also be written with the help of Ne (Neon) as well. as Neon completes its all shells or orbits and the electronic configuration can also be written as: [Ne] 3s23p2.
Silicon Number of Valence Electrons
A valence electron is defined as the number of electrons in the outer shell. Here the numbers of electrons in the outer shell are 4 as in the outer or 3rd shell it has 4 elements.
Ground State Electron Configuration For Silicon
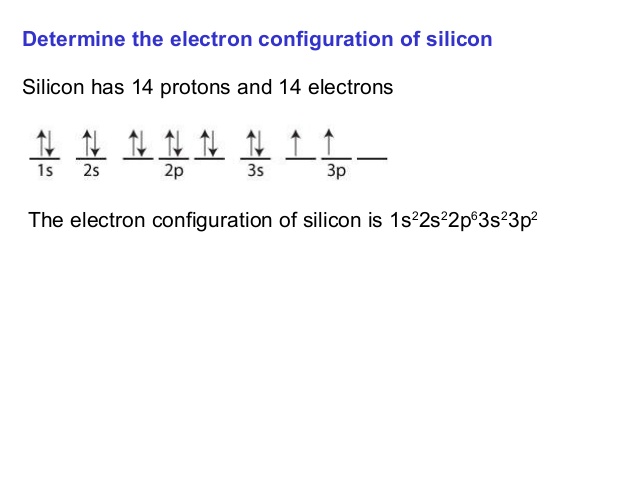
On the basis of pair distribution ground state electron configuration is defined. And this can be well defined with the help of an orbital diagram in which single box consists of a pair of electrons. The electron configuration for Si is 1s22s22p63s23p2.
What is the Electron Configuration of Silicon
Electron configuration is the number of electrons present in each orbit of atom or molecules. In the case of silicon, the electrons are present in 3 shells that are 2, 8, 4. And it’s electron configuration is 1s22s22p63s23p2
How Many Valence Electrons are in Silicon
There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4.
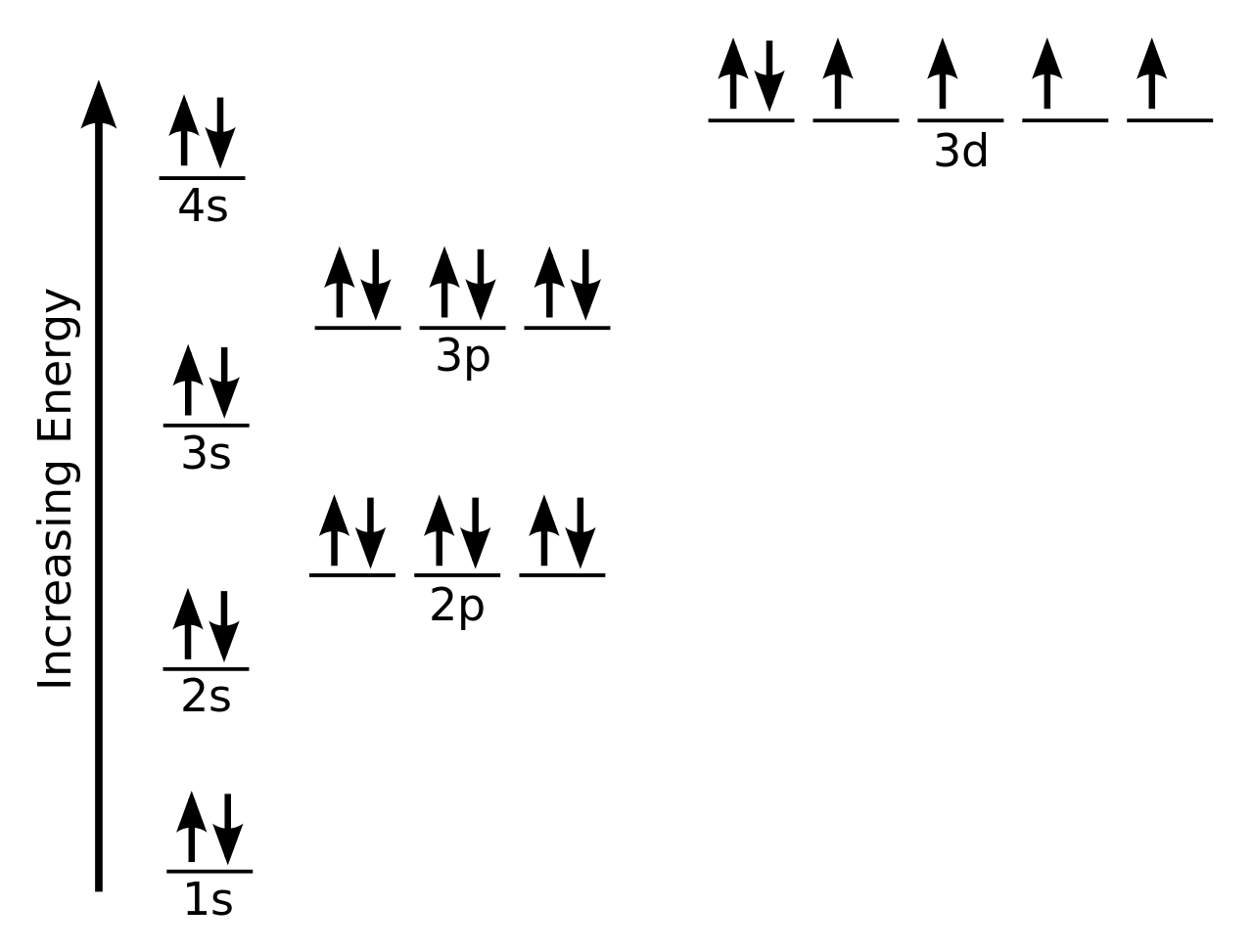
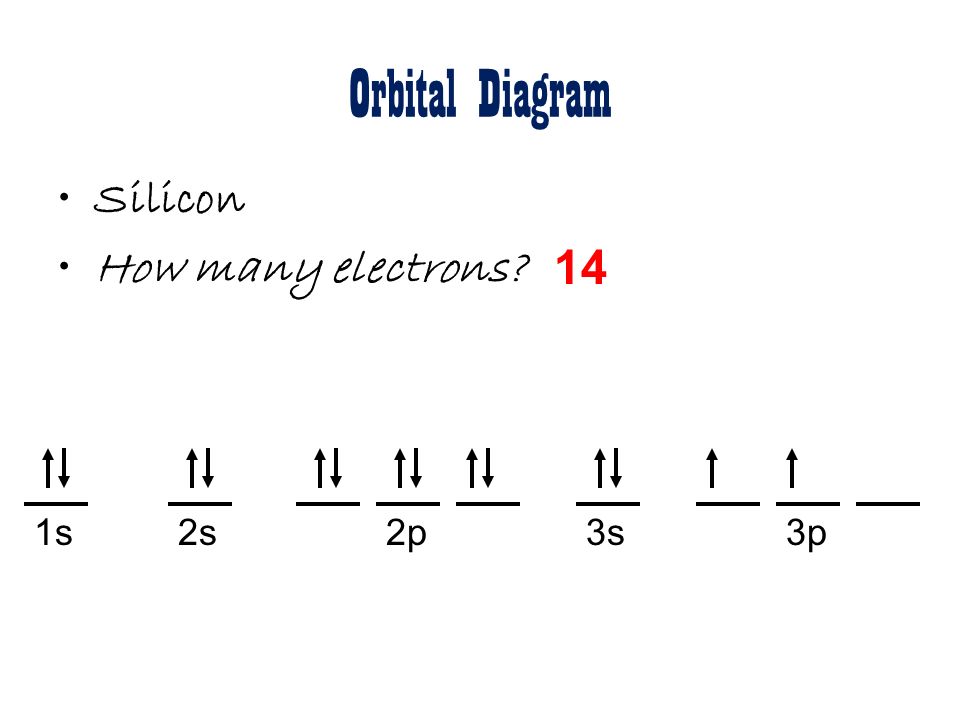
Silicon Orbital Diagram
Orbit diagram consists of a pair of electrons of the atom in the box i.e.
Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.