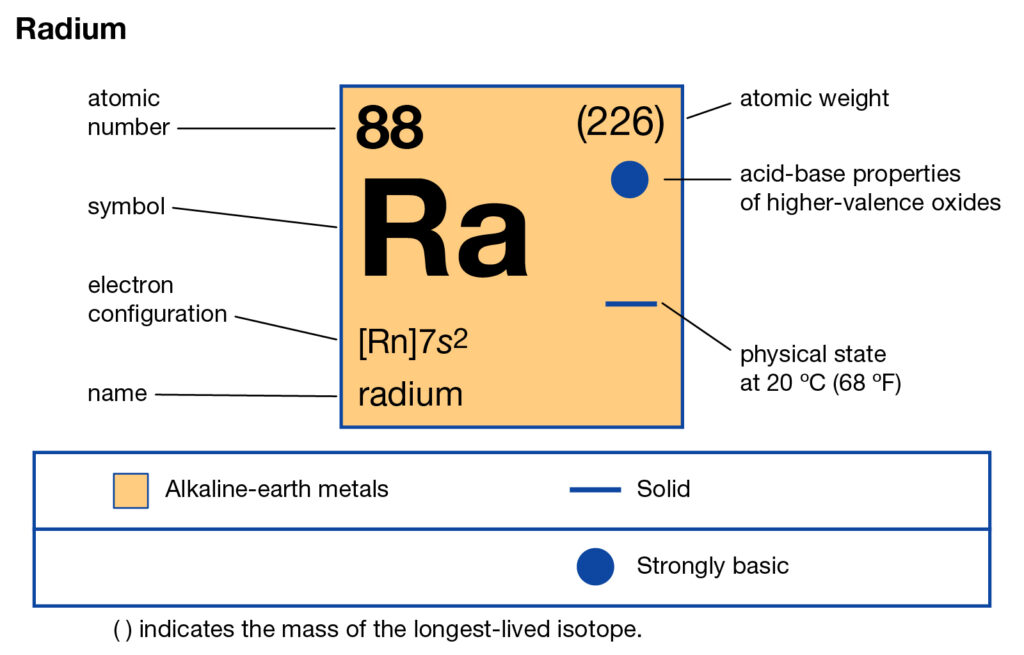
All the chemistry scholars can here read about the Radium valence electrons. We are providing a systematic study of the element. Radium is a chemical element with the atomic number 88 in the periodic table. The element has the representative symbol of Ra and belongs to block s. Radium further falls in the category of Alkaline earth metals. It has the structure of white silvery metal with some reactive properties.
How many valence electrons does Radium have?
It easily reacts to nitrogen and has highly radioactive isotopes. Radium 226 is its highly stable isotope with a life span of around 1600 years.
Radium occurs in the environment in the form of a byproduct of other elements. It basically occurs in the uranium and uraninite ores. Some minor quantity of Radium is extracted from the thorium minerals as well. So, Radium has enough availability in the environment for ample usage.
Radium is a highly radioactive element hence it has very toxic effects. It may cause some lethal adverse effects on human health. Human exposure to Radium may cause the bloodstream to retain the radioactive effects. It may hence cause life-threatening side effects on humans and other living species.
Being the radioactive chemical element Radium has similar applications. Its role is gradually increasing in the domain of atomic and molecular research. It’s further useful to use as a decent source of radiation for some special causes. The element is still in its research phase to offer more practical commercial usage.
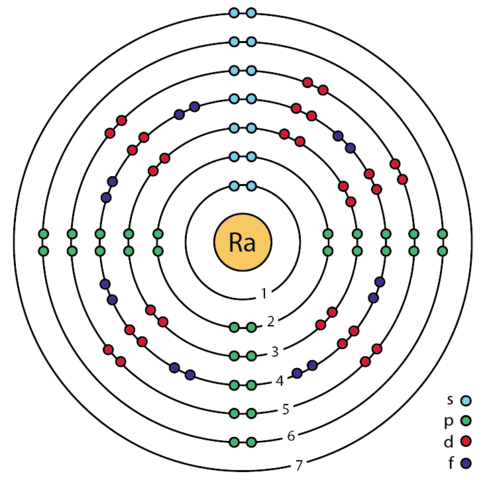
Radium Valence Electrons Dot Diagram
Lewis dot structure is the potent tool for studying the Radium valence electrons. The structure is useful to represent the numbers of valence electrons of atoms for any element.
It further helps in explaining the interaction and binding of valence electrons. Consequently, you can figure out the bonding pattern or outcome of valence electrons.
Valency of Radium
Radium has 2 valence electrons hence it has a valency of 2. Valency states the combining capacity of the Radium chemical element.
It’s useful to understand how the atoms of the element will react with other elements. Almost all the chemical elements carry their respective valency.