Mass of a Proton Neutron and Electron: The mass of fundamental particles plays a pivotal role in our understanding of the universe’s building blocks. In this exploration, the focus lies on the mass of three crucial subatomic particles: the proton, neutron, and electron. These particles constitute the foundation of matter, with protons and neutrons residing in atomic nuclei, while electrons orbit around them.
Understanding their masses is fundamental to comprehending the structure and behavior of atoms. This article delves into the distinct masses of these particles and sheds light on their significance in the realm of particle physics and cosmology.
What is The Mass of a Proton Neutron and Electron?
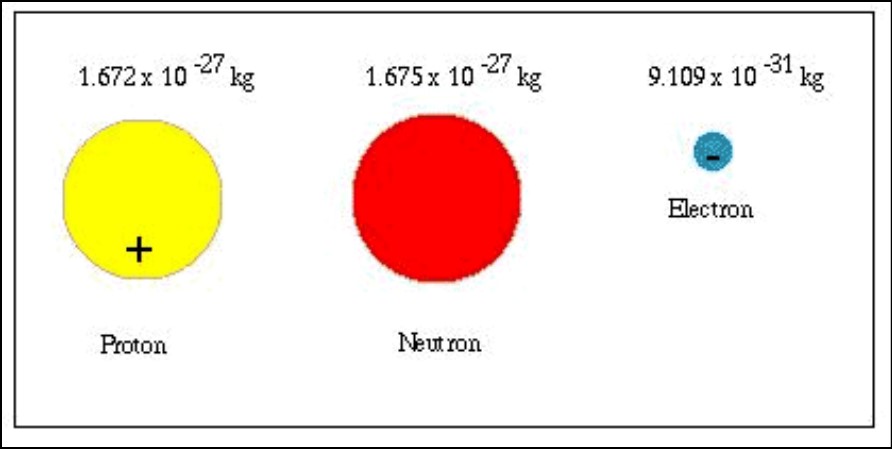
Proton: The proton, one of the fundamental particles that make up the atom’s nucleus, along with the neutron. It carries a positive electric charge and is denoted by the symbol “p” or “p^+”. The mass of a proton, approximately 1.67262192 × 10^-27 kilograms (kg). In atomic mass units (u), a proton’s mass is approximately 1.00727 u. The proton’s mass was significant in determining the overall mass of an atom since it contributes significantly to the atomic mass.
Neutron: The neutron is another fundamental particle found in the atomic nucleus, and it has no electric charge, making it electrically neutral. The symbol used to represent a neutron is “n.” The mass of a neutron is slightly larger than that of a proton, approximately 1.674927471 × 10^-27 kg (1.00866 u). Neutrons play a crucial role in stabilizing the atomic nucleus through the strong nuclear force, which counters the electromagnetic repulsion between protons.
Electron: The electron is a subatomic particle that orbits around the atomic nucleus. It carries a negative electric charge and is represented by the symbol “e^−”. Compared to protons and neutrons, electrons have a much smaller mass. The mass of an electron is approximately 9.10938356 × 10^-31 kg (0.00054858 u), which is roughly 1836 times lighter than a proton or neutron. Despite their small mass, electrons are vital in determining the chemical and electrical properties of atoms and molecules.
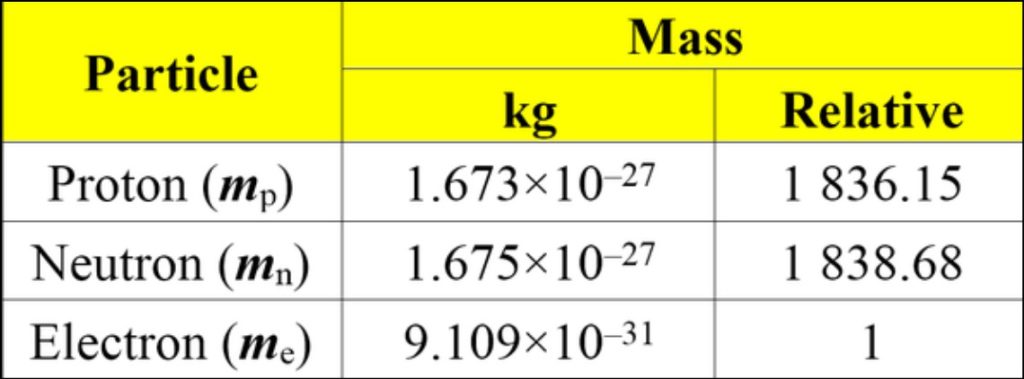
Comparison: Protons and neutrons have masses of approximately 1.67262192 × 10^-27 kg and 1.674927471 × 10^-27 kg, respectively, while electrons have a much smaller mass of about 9.10938356 × 10^-31 kg. As a result, protons and neutrons roughly 1836 times more massive than electrons. This significant difference in mass plays a fundamental role in shaping the behavior and interactions of these particles within atoms and the universe.
Proton Mass and Charge
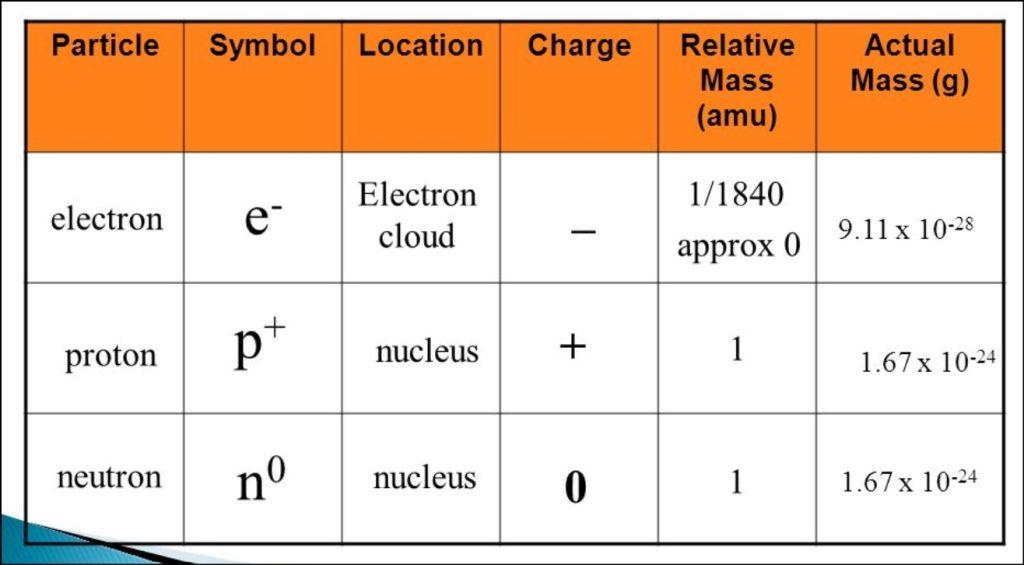
The proton is a subatomic particle found in the atomic nucleus and plays a crucial role in defining the properties of chemical elements. It carries a positive electric charge of approximately +1 elementary charge, which equal to 1.602176634 × 10^-19 coulombs (C). This charge is denoted by “e” in physics equations and represents the smallest unit of electric charge. The proton’s charge fundamental in establishing the atom’s overall charge, as the number of protons in an atom’s nucleus determines its atomic number, defining the element’s identity.
In addition to its electric charge, the mass of a proton is significant in understanding the structure of matter. As previously mentioned, a proton’s mass is approximately 1.67262192 × 10^-27 kilograms (kg). This mass is essential for calculating the total mass of an atom, as protons contribute substantially to the atom’s overall mass, along with neutrons. Protons are held together within the nucleus through the strong nuclear force, which overcomes the electromagnetic repulsion between positively charged protons.
The concept of charge and mass of protons has implications beyond atomic structure. In high-energy physics and particle accelerators, protons are commonly used as projectiles due to their positive charge. When accelerated to high speeds, they can collide with other particles or nuclei. Leading to the discovery and study of various subatomic particles and fundamental forces.
Overall, the proton’s mass and charge critical characteristics that define its role in atomic structure, chemistry, and high-energy physics experiments. Understanding these properties is essential for unraveling the mysteries of the subatomic world and the universe’s fundamental building blocks.
Proton Neutron Electron Mass
The masses of protons, neutrons, and electrons are crucial pieces of information in understanding the structure of matter and the fundamental forces that govern the universe. As previously mentioned, the mass of a proton is approximately 1.67262192 × 10^-27 kilograms (kg), the mass of a neutron is about 1.674927471 × 10^-27 kg, and the mass of an electron is approximately 9.10938356 × 10^-31 kg. Comparing these masses, we can see that protons and neutrons have very similar masses, both being roughly 1836 times heavier than electrons.
The mass of a proton and neutron is relatively large compared to the electron, and this difference in mass plays a vital role in determining the stability of atoms. The protons and neutrons in the nucleus contribute to most of the atom’s mass and are held together by the strong nuclear force, overcoming the electromagnetic repulsion between the positively charged protons.
In contrast, electrons, with their significantly smaller mass, orbit the nucleus in energy levels. These negatively charged electrons are bound to the positively charged nucleus by the electromagnetic force. The arrangement of electrons in different energy levels defines an atom’s chemical properties and determines how it interacts with other atoms to form molecules.
The study of particle masses also extends beyond the realm of atoms and molecules. Particle physicists use this knowledge to explore the subatomic world, including high-energy particle collisions and the existence of exotic particles like quarks, gluons, and neutrinos. Understanding particle masses is a crucial step in unraveling the mysteries of the universe and its fundamental building blocks.
Proton and Electron Mass
The mass of protons and electrons is a fundamental property that differentiates these subatomic particles. As mentioned earlier, the mass of a proton is approximately 1.67262192 × 10^-27 kilograms (kg). The mass of an electron is significantly smaller, around 9.10938356 × 10^-31 kg. This makes the proton approximately 1836 times more massive than the electron. The disparity in their masses is a crucial factor that influences the behavior of atoms and their chemical interactions.
In atomic structures, protons and electrons play contrasting roles. Protons, found in the nucleus, are responsible for determining an atom’s identity, as the number of protons in an atom’s nucleus corresponds to its atomic number. In contrast, electrons orbit the nucleus in energy levels, participating in chemical reactions and the formation of chemical bonds. The balance between the positive charge of protons and the negative charge of electrons keeps an atom electrically neutral.
Moreover, the mass of an electron is so much smaller compared to protons and neutrons that, for most practical purposes, it is considered negligible when calculating the total mass of an atom. Instead, the atomic mass is primarily determined by the sum of protons and neutrons within the nucleus.
In particle physics, the mass of the electron is a significant parameter in various experiments and theoretical models. For instance, the electron’s mass influences the behavior of electrons in particle accelerators and determines the energies required for specific interactions.
In conclusion, the mass of a proton and an electron greatly differs, and this disparity has far-reaching consequences in the realm of atomic and particle physics. The mass of protons contributes to the overall mass of an atom, while electrons’ negligible mass allows them to participate actively in chemical processes and define the physical and chemical properties of elements.