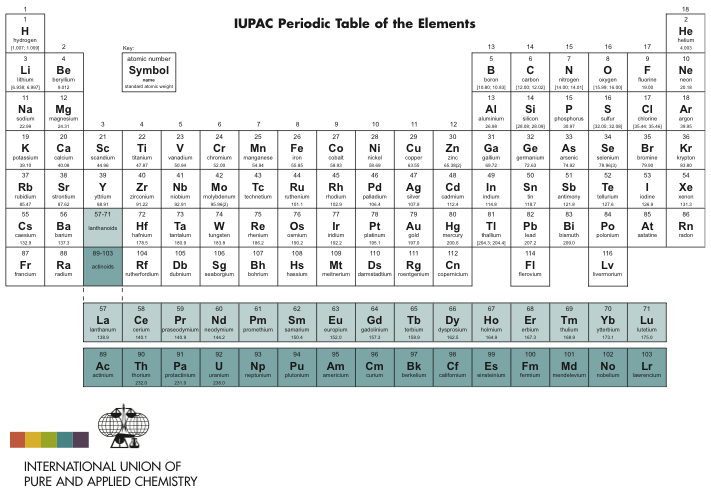
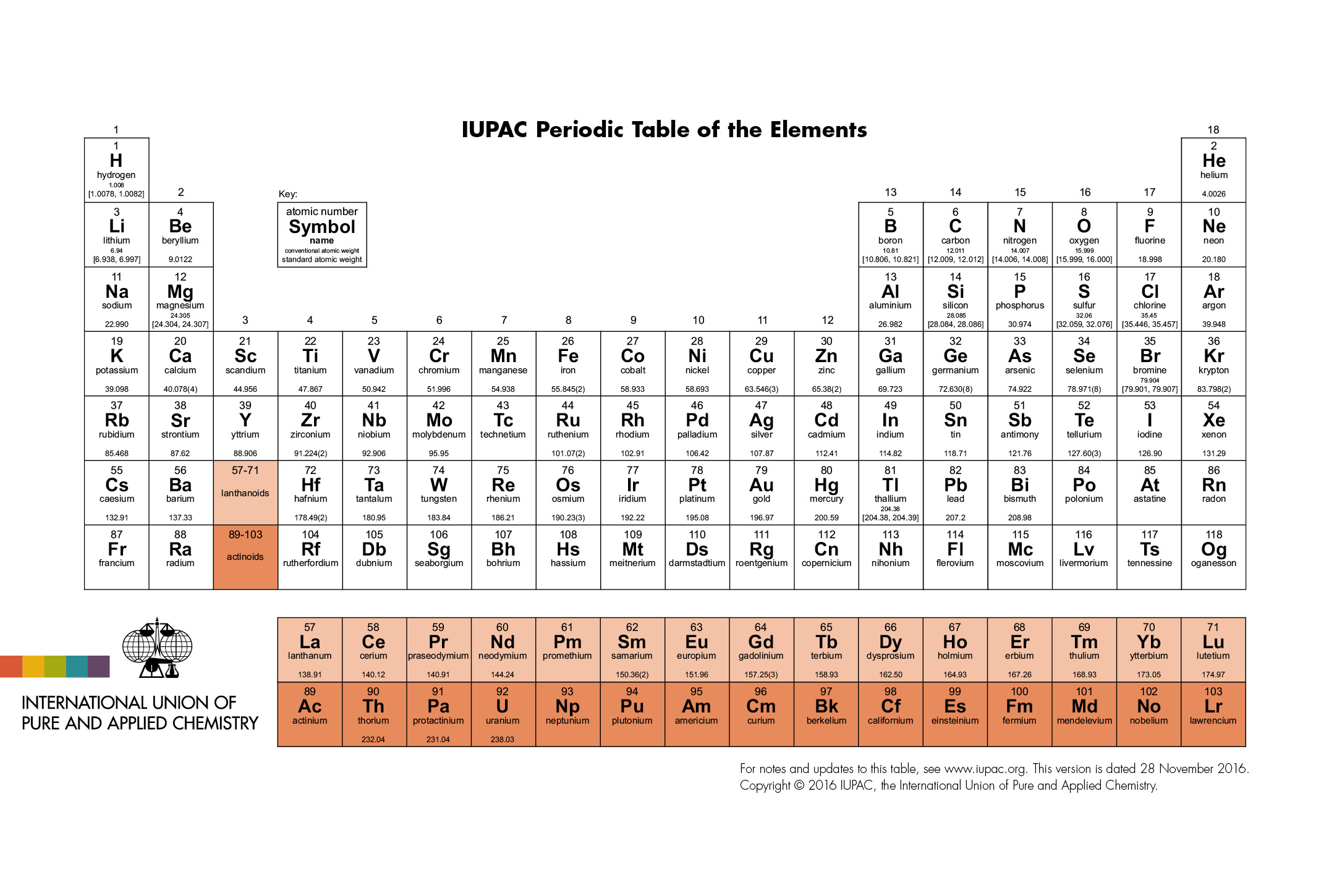
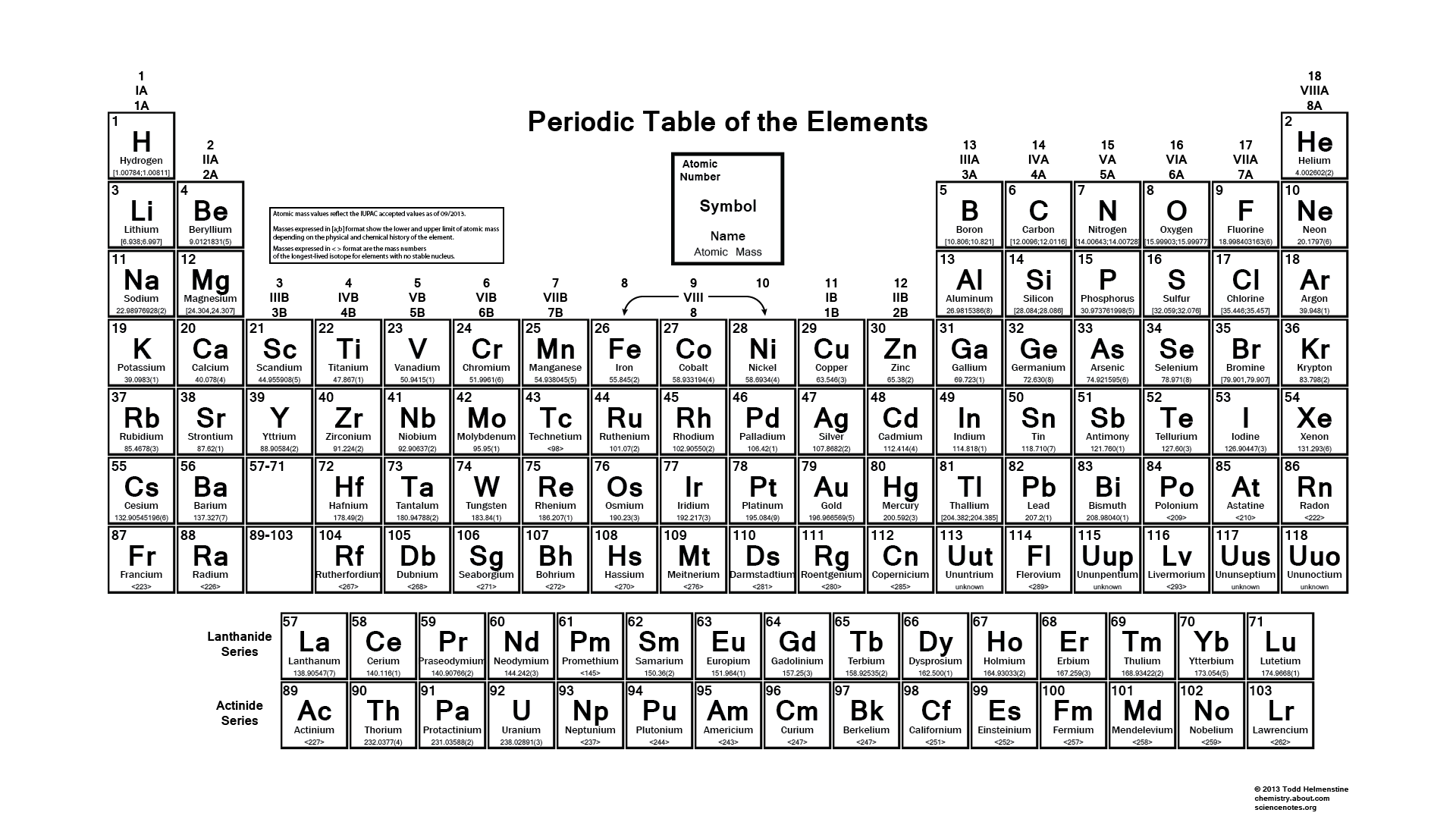
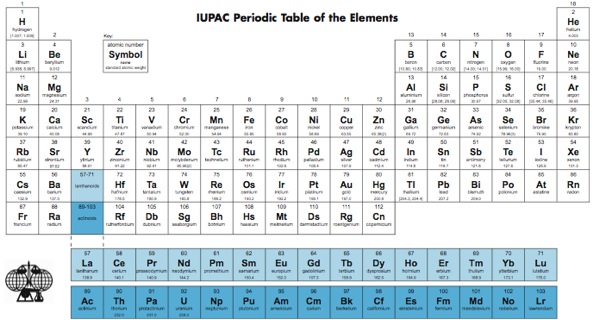
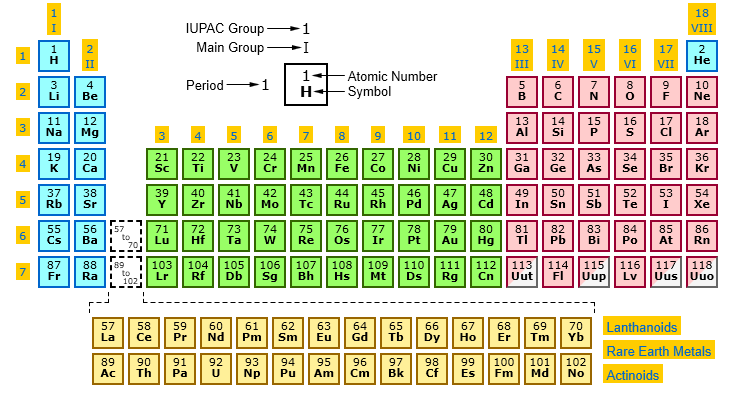
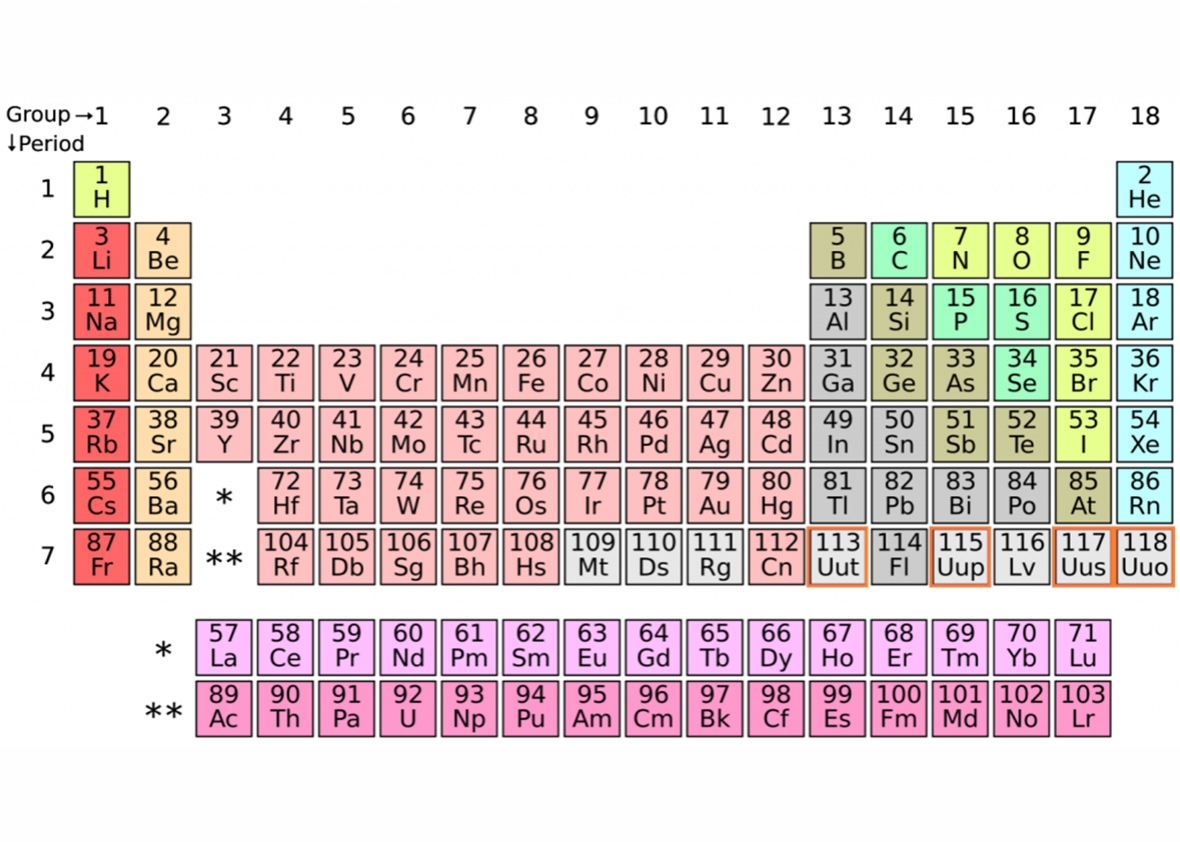
IUPAC Periodic Table: IUPAC is the short form for International Union of Pure and Applied Chemistry. It is the international scientific organization that is not affiliated by any government. The IUPAC Strive its aim to advance chemistry by setting global standards for symbols, names and units of various chemical elements.
IUPAC Periodic Table of Elements
The IUPAC Periodic Table of Elements, often referred to as simply the “Periodic Table,” is a tabular arrangement of chemical elements organized by their atomic number, electron configuration, and recurring chemical properties. This iconic representation provides a systematic way to understand the relationships between various elements and predict their behaviors in chemical reactions. Dmitri Mendeleev is credited with the creation of the first widely recognized periodic table in the late 19th century. The modern IUPAC version has undergone several revisions to accommodate new discoveries and insights into the nature of atoms and their properties.
The table organized into periods (rows) and groups (columns). Elements in the same group share similar chemical properties due to their similar electron configurations, leading to analogous valence electron structures. Periods represent the successive addition of electrons to higher energy levels, while groups signify elements with the same number of valence electrons. The periodic table’s design allows scientists to identify trends in properties like atomic radius, electronegativity, and ionization energy, which aid in understanding an element’s reactivity and potential applications in various fields.
In summary, the IUPAC Periodic Table of Elements serves as a cornerstone of chemistry, enabling scientists to comprehend the relationships between elements and their properties, thus forming the foundation for various scientific disciplines and applications. Learn Periodic Table of Elements With Names and Symbols here.
International Union of Pure and Applied Chemistry Periodic Table PDF
The International Union of Pure and Applied Chemistry (IUPAC) provides various resources to facilitate the understanding and dissemination of scientific knowledge, including the Periodic Table of Elements. To enhance accessibility and convenience, IUPAC offers a downloadable PDF version of the Periodic Table on its official website. This PDF version encompasses the latest updates and additions to the elements, ensuring that educators, researchers, and students have access to accurate and up-to-date information.
The PDF version of the IUPAC Periodic Table typically includes elements arranged according to their atomic number, accompanied by elemental symbols and names. Additionally, important information such as atomic mass, electron configuration, and standard atomic weights may also included. This PDF resource serves as a valuable tool for educators and researchers who require a tangible reference for their work and presentations.
To access the IUPAC Periodic Table PDF, interested individuals can visit the IUPAC website, navigate to the Periodic Table section, and locate the downloadable PDF file. This resource underscores IUPAC’s commitment to advancing the field of chemistry and promoting the accurate representation of elemental properties.
IUPAC Periodic Table 2018
The IUPAC Periodic Table underwent a significant update in 2018, which introduced changes to the ways atomic weights expressed and determined. Prior to this update, atomic weights were often represented as single values, which didn’t account for the natural isotopic variations of elements. The 2018 revision, however, implemented a more nuanced approach by presenting atomic weights as intervals, reflecting the isotopic distribution of an element in nature.
For example, the atomic weight of an element now presented as a range to indicate the variations caused by differing proportions of isotopes. This revision acknowledges that elements found in nature often mixtures of isotopes with slightly different atomic masses. The IUPAC 2018 update aimed to provide a more accurate representation of an element’s atomic weight while considering the variations observed in naturally occurring samples.
This change significant because it ensures that the periodic table remains aligned with the latest scientific knowledge and technological advancements. Researchers and educators can now more precisely convey the complexities of elemental properties and their isotopic distributions, leading to a better understanding of the natural world.
IUPAC Periodic Table with Names
The IUPAC Periodic Table with Names an extended version of the traditional periodic table that includes the names of elements, making it easier for learners to associate symbols with their corresponding element names. While the standard periodic table uses elemental symbols, this version caters to students, educators, and individuals who less familiar with the symbols but wish to learn about the elements in detail.
For example, instead of simply displaying “Fe” for iron, the IUPAC Periodic Table with Names would have “Iron” alongside the symbol. This addition aids in the learning process, especially for beginners who just starting their exploration of chemistry. It also helps bridge the gap between the scientific community and the general public by making the information more accessible.
The inclusion of element names on the periodic table contributes to effective science communication and education, promoting a broader understanding of the fundamental building blocks of matter. It’s particularly beneficial for students who new to chemistry and in the early stages of developing their knowledge and appreciation of the subject. This version of the periodic table serves as a valuable tool in classrooms, laboratories, and public outreach events, enriching the learning experience and fostering a deeper connection with the world of science.
Atomic Number of IUPAC Periodic Table
The atomic number a fundamental property used to uniquely identify each chemical element within the IUPAC Periodic Table. It corresponds to the number of protons found in the nucleus of an atom of that element. This property defines an element’s identity and its position within the periodic table’s structure.
Elements arranged in ascending order of atomic number, which in turn determines their position within periods (rows) and groups (columns) of the table. The atomic number a crucial factor in determining an element’s chemical behavior, as it dictates the number of electrons orbiting the nucleus. Elements with the same atomic number considered isotopes of each other, sharing the same number of protons but differing in the number of neutrons.
In the context of the periodic table, the atomic number provides insight into the relationships between elements and their properties. The arrangement of elements by increasing atomic number results in a pattern of recurring chemical behaviors and trends that scientists can use to predict and understand the behavior of different elements. This systematic organization allows for the classification and categorization of elements based on their shared characteristics, fostering a deeper understanding of the natural world and the building blocks of matter.