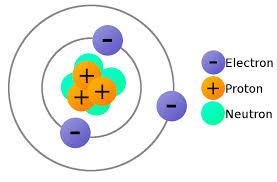
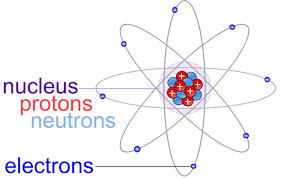
Proton Charge and Mass: The atomic structure consists of a nucleus consisting of protons and neutrons and electrons revolving around its orbital shell. Protons are positively charged sub-atomic particle which makes up most of the mass of an atom. They define the property of an element as the number of protons in an atom makes up its atomic number. In any given atom, the number of protons in the nuclei is always the same.
What is Proton Charge and Mass?
Protons are subatomic particles that are found in the nucleus of atoms. They carry a positive electrical charge and have a relatively high mass compared to other subatomic particles.
Here are the key characteristics of protons:
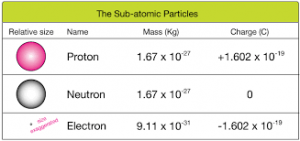
- Charge: The charge of a proton is positive. It has a fundamental charge of +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs. The charge of a proton is equal in magnitude but opposite in sign to the charge of an electron, which is negative.
- Mass: The mass of a proton is approximately 1.67 x 10^-27 kilograms (kg) or 1.67 x 10^-24 grams (g). It is roughly 1,836 times more massive than an electron. The mass of a proton is similar to the mass of a neutron, another subatomic particle found in the nucleus of atoms.
The charge and mass of a proton are fundamental properties that play a crucial role in the structure and behavior of atoms. The positive charge of protons contributes to the overall charge of the nucleus, which, in turn, determines the atom’s chemical properties and interactions with other atoms. Additionally, the mass of protons affects the overall mass of an atom, influencing its physical properties, such as density and atomic weight.
Understanding the charge and mass of protons is fundamental to the field of particle physics and is essential in our comprehension of atomic and nuclear structures, as well as the fundamental forces that govern the behavior of matter. Learn more:- Valency For All the Elements, Neutron Symbol Mass and Charge.
Why Do Protons and Electrons have Charges?
Protons and electrons have charges because they are elementary particles that possess fundamental properties, including electric charge. The existence of electric charge is a fundamental property of matter and is related to the fundamental forces in the universe.
The charge of a particle determines how it interacts with electric and magnetic fields, as well as with other charged particles. The charge of an object can positive, negative, or neutral. Protons and electrons have opposite charges, with protons carrying a positive charge and electrons carrying a negative charge.
The origin of these charges lies in the properties of quarks, which subatomic particles that make up protons and neutrons. Protons composed of two up quarks and one down quark, while electrons considered elementary particles and not made up of smaller particles.
The positive charge of a proton arises from the electric charge of the up and down quarks within it. Up quarks have a charge of +2/3 (in units of elementary charge), while down quarks have a charge of -1/3. When combined in the proton, the total charge adds up to +1.
Similarly, electrons have a negative charge. Electrons not made up of smaller particles and considered to elementary. The exact nature of the charge of an electron is not yet fully understood and is a topic of ongoing research in particle physics.
The presence of charges in protons and electrons allows for the existence of electric and magnetic fields, and it is through the interaction of these charged particles that various phenomena, such as electricity, magnetism, and chemical bonding, occur in the natural world.
How Much Charge Does a Proton Have?
A proton carries a positive electric charge of approximately +1 elementary charge. The elementary charge is denoted as “e” and is approximately equal to 1.602 x 10^-19 coulombs. Therefore, a proton has a charge of approximately +1.602 x 10^-19 coulombs.
It’s important to note that the elementary charge is a fundamental physical constant that represents the magnitude of electric charge carried by elementary particles, such as protons and electrons. The charge of a proton, which is +1 elementary charge, is equal in magnitude but opposite in sign to the charge of an electron, which is -1 elementary charge.
The electric charge of a proton is a fundamental property that plays a significant role in the behavior of particles and the structure of atoms. The positive charge of protons is one of the factors that contributes to the overall charge of atomic nuclei and determines the interactions of atoms with other charged particles and electromagnetic fields. As we have discussed, a proton has a positive charge of +1.
Charge of a Proton and Electron
The charge of a proton is +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs. It carries a positive charge.
On the other hand, the charge of an electron is -1 elementary charge, which is the same magnitude as that of a proton but opposite in sign. The charge of an electron is also approximately 1.602 x 10^-19 coulombs, but it carries a negative charge.
It is important to note that the elementary charge is a fundamental physical constant in physics and serves as the basic unit of electric charge. The charge of an electron and a proton fundamental properties of these particles and play a significant role in understanding the behavior of matter and electromagnetic interactions.