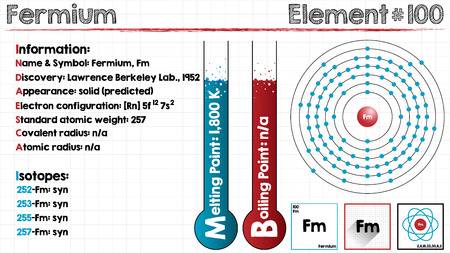
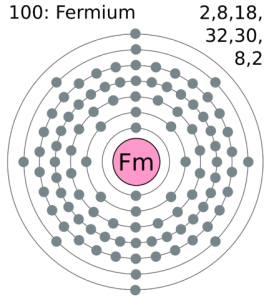
All our scholar’s readers can here explore the Fermium valence electrons for their systematic understanding of the element. Here in the article, we are going to cover up the systematic study for the valence and valence electrons of the Fermium. Our objective is to provide our readers with useful information about the element so that they can process the knowledge of this element. Fermium is the chemical element in the domain of chemistry from its periodic table.
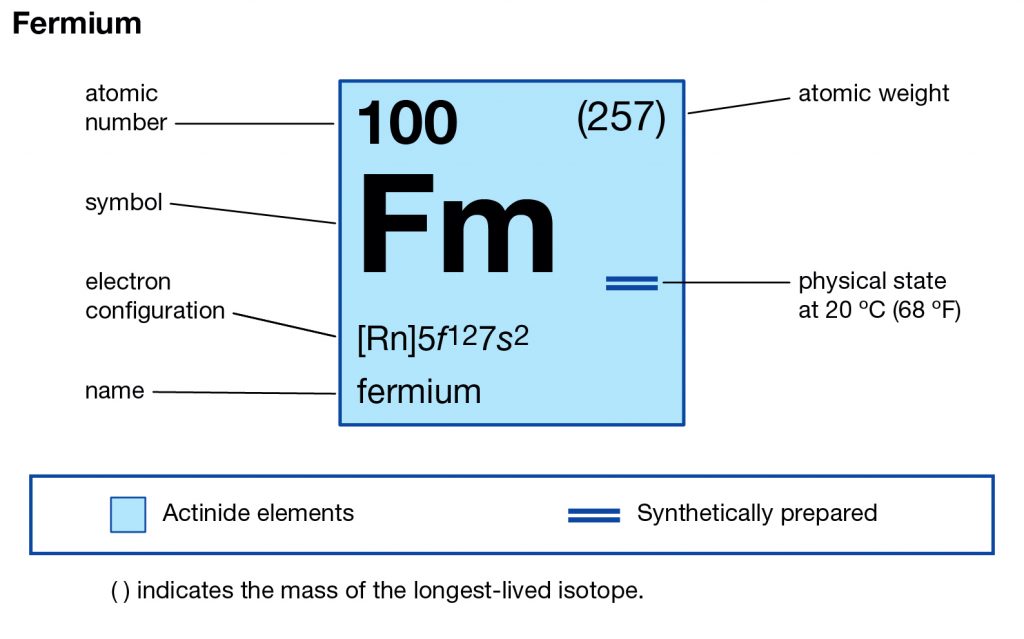
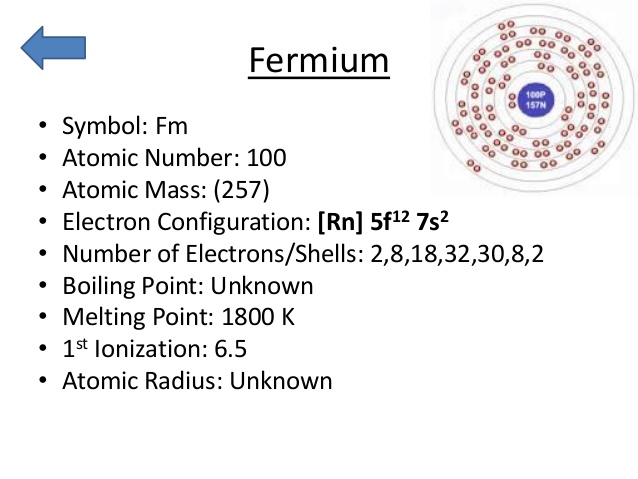
The element is purely synthetic in nature and comes with its atomic number of 100 and the symbol of Fm. The element belongs to the category of the Actinide series of the elements and has no free form of occurrence within nature. One can create this element by the bombardment of the lighter elements of the neutrons. Since the element is radioactive therefore it’s not safe for human exposure and remains mostly in the laboratory.
How Many Valence Electrons Does Fermium Have?
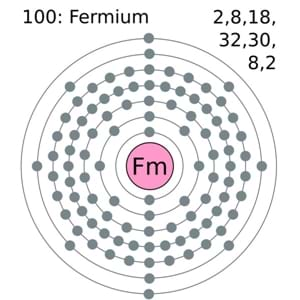
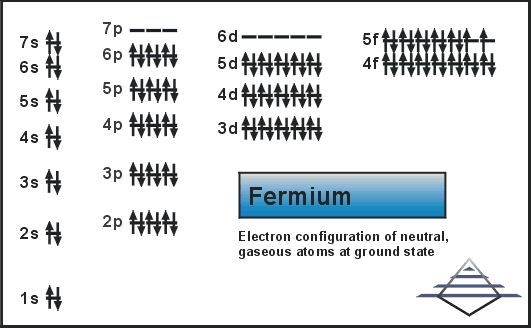
Fermium comes with the 4 (Four) valence electrons in the outer shell which are responsible for the combining activities of the element. Valence electrons refer to the number of electrons that are available in the outer shell of the element. These electrons are useful in conducting further research on the element and finding its other chemical properties.
Fermium Valence Electrons Dot Diagram
Lewis dot diagram comes highly handy in the representation of the valence electrons of the Fermium. This diagram is useful for all those scholars who are struggling to figure out the valence electrons of the element. In this diagram basically, they will find the numbers of dots in the molecule of the element that is equal to the numbers of valence electrons. The diagram can also state that whether these valence electrons are nuclear or occur in the combination form.
Valency of Fermium
Fermium has the valency of (3,2) which is the combining capacity of the element. It means that the element may gain or lose these electrons to achieve a state of stable election configuration. The valency of the element is as significant as the valence electrons of the element