Electron Configuration For Fermium: Fermium is a synthetic chemical element that has a symbol Fm. The atomic number of fermium is 100. It is an actinide and the heaviest element that is formed by bombardment neutron of lighter elements, and also the last element that is prepared in macroscopic quantities.
Electron Configuration For Fermium
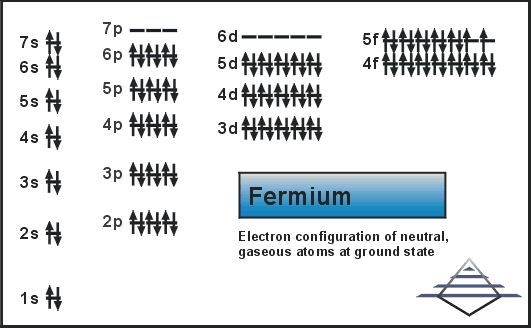
It was discovered in 1952 in the debris of the first hydrogen bomb explosion. It was named after Enrico Fermi, one of the pioneers of nuclear physics. Today we are here to share the information about the electron configuration of the Fm.
If you guys are also here to get the information about this element then you are at the right place. Please go through the full post below.
What is the Electron Configuration of Fm
How Many Valence Electrons does Fermium Have
Fermium has five valence electrons in its outer shell.
Fermium Number of Valence Electrons
There is five valence electron in the outer shell of the Fm.
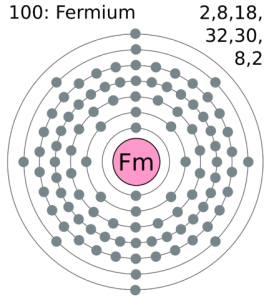
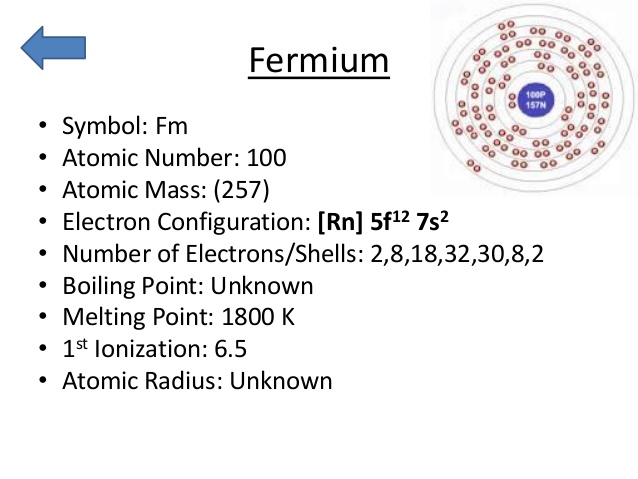
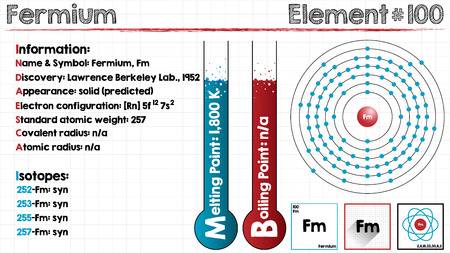
Leave a Reply