Get to understand the Chromium electron configuration and other significant properties of the element here in the article ahead. The article will describe the other basic information along with the other significant aspects of the Chromium to all the enthusiasts.
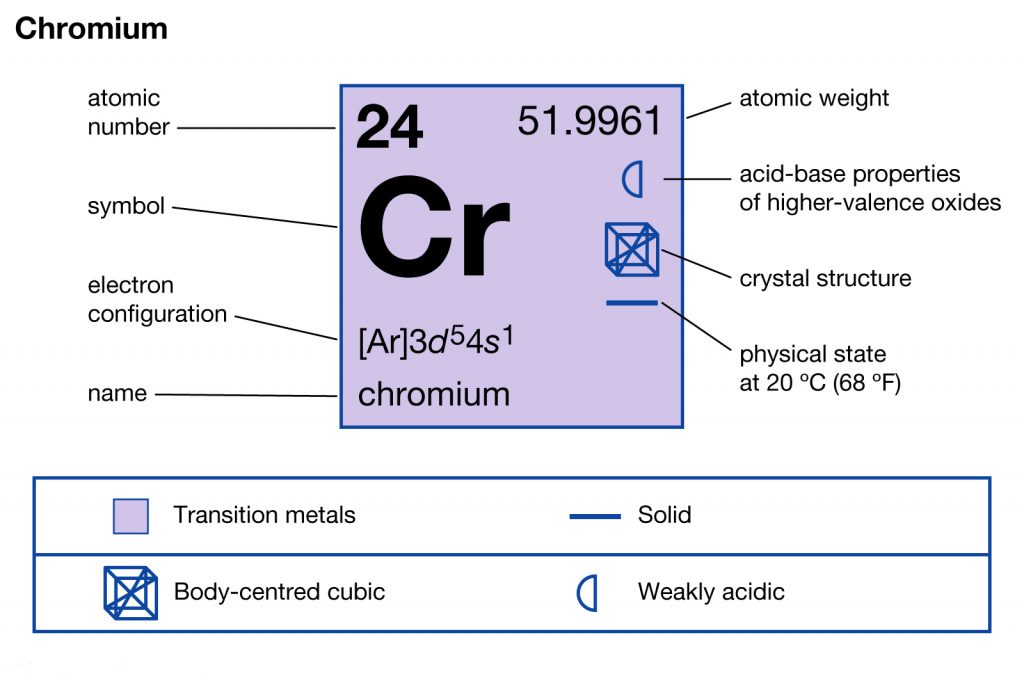
Well, Chromium is basically a chemical element in the domain of chemistry that’s studied by scholars in the periodic table. It has the representation symbol of Cr along with the atomic number 24. The chemical element belongs to the category of group 6 and is the first chemical element of the same category.
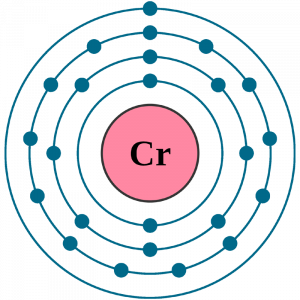
Chromium Electron Configuration
It’s one of those chemical elements that have very high practical relevance in the industrial domain across the world. For the same reason, it’s one of the highly popular and demanded chemical elements in the world. You can easily witness its significance in several local or international production factories such as in the steel industries etc.
[Ar] 3d⁵ 4s¹
Well, the term electron configuration is nothing new to the scholars of chemistry, however, it may a bit technical to others. Chromium electron configuration in an easy language is basically the process in which the chemical element distributes its electrons in a given system of orbitals. This is a mandatory scientific process in the domain of chemistry and every chemical element has to go through this process.
What Is The Electron Configuration of Chromium?
So, with the same process, the electron configuration of the Chromium will actually be 1s22s22p63s23p44s23d9. This is the standard electron configuration of the Chromium after applying the election configuration formula.
How Many Valence Electrons Does Chromium Have?
Well, we believe that just knowing the answer of Chromium electron configuration in itself is not enough. Rather you should be aware of the actual derivation method of the answer. The derivation concept for the electron configuration brings clarity to scholars.
Chromium Number of Valence Electron
So, keeping the same in mind we here have the derivation concept of Chromium electron configuration.
- We know that Chromium has an atomic number of 24 so the first two electrons of Chromium will shift to the 1s orbital.
- The 1s orbital can hold only 1 electron therefore the other 2 electrons will move on to the 2s orbital.
- The next six electrons will then move to the 2p orbital.
- In a similar manner, the 3s orbital will have 2 electrons and then 3p will assimilate the 6 further electrons.
- for the 4s orbital, we will have the 2 electrons and then the remaining electrons will be held by the 3d orbitals.
- In the end, this whole distribution process will what make the Cr electron configuration 1s22s22p63s23p44s23d9.
So, we hope this clarification of the Cr electron configuration will make sense to our scholars. They can also share the same information with their classmates or other friends.