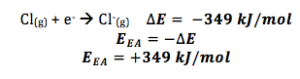What is Meant By Electron Affinity Equation? Electron affinity is the amount of energy changed (in kJ/mole) when an electron is introduced to a neutral atom in the gaseous state. The resultant reaction may be endothermic or exothermic and produces a negative ion. In other words, it is the capability of the positive nucleus of an atom to accept another negative electron within its orbital shell.
What is Meant by Electron Affinity?
Electron affinity refers to the energy change that occurs when a neutral atom in the gas phase gains an electron to form a negatively charged ion, known as an anion. It is a measure of the attraction between the incoming electron and the nucleus of the atom.
The electron affinity of an atom is typically expressed as a negative value or in units of energy (e.g., electron volts or kilojoules per mole) because energy is released when an atom gains an electron. The more negative the electron affinity value, the higher the attraction between the atom and the electron.
The electronic affinity equation can be described in two stages. In the first stage, electron affinity is the amount of energy released to form 1 mole of negative ions.
X(g)+e−→X−(g)
During the second stage, when the incoming electron is introduced to the negative ion, the amount of energy required is more than the energy released. Hence the equation is :
X−(g)+e−→X2−(g)
When an atom gains an electron, the electron occupies an orbital around the nucleus. This process releases energy if the attraction between the electron and the nucleus is strong. However, if the atom already has a strong electron-electron repulsion due to its electron configuration, it may require additional energy to overcome this repulsion and accept an electron. In such cases, the electron affinity can be positive, indicating that energy is required to add an electron.
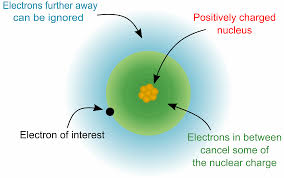
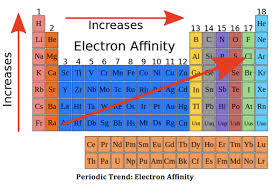
Electron affinity is influenced by various factors such as atomic size, nuclear charge, and electron configuration. Elements with smaller atomic size and higher effective nuclear charge tend to have higher electron affinities because their electrons are closer to the nucleus and experience a stronger attraction. Check out other posts:- Electron Affinity Trend, Electron Cloud Definition, Electronegativity Series.
How To Write Electron Affinity Equation
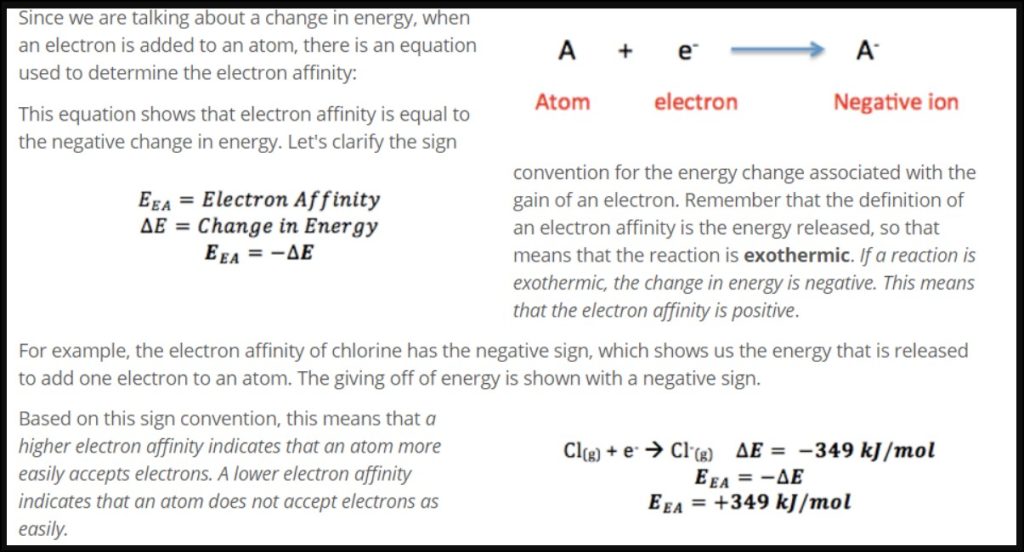
The electron affinity (EA) of an atom or molecule is defined as the energy change that occurs when an electron is added to a neutral atom or molecule in its gaseous state. The electron affinity is typically expressed as a negative value because energy is released when an electron is added. The general equation for electron affinity can be written as:
A + e⁻ → A⁻ + energy
In this equation, A represents the atom or molecule to which the electron is being added, e⁻ represents the electron, A⁻ represents the resulting negatively charged ion, and “energy” represents the energy released during the process.
It’s important to note that the electron affinity can vary depending on the specific atom or molecule. Some atoms or molecules readily accept electrons and have a high electron affinity, while others have a low electron affinity or may even release energy when an electron is added.
During the second stage of electronic affinity, when the negative ion is introduced to another electron, the amount of energy required to incorporate another electron is greater than the energy released. Hence the atom loses its energy and in the process undergoes an exothermic reaction.
The second stage chemical reaction can be depicted through the following equation :
X−(g)+e−→X2−(g)
Keep in mind that the electron affinity equation is a general representation, and there may be additional factors or considerations for specific elements or compounds. Additionally, the electron affinity values are often experimentally determined and can be found in reference books or databases.
First Electron Affinity Equation
The first electron affinity (EA) is defined as the energy change that occurs when a neutral atom in the gas phase accepts an electron to form a negatively charged ion. It is represented by the equation:
A(g) + e⁻ → A⁻(g) + energy
where A represents the atom, (g) denotes the gaseous state, e⁻ represents an electron, A⁻ represents the resulting negatively charged ion, and “energy” represents the energy released or gained during the process.
The first electron affinity is typically expressed as a negative value because energy is released when an atom accepts an electron, indicating the formation of a more stable ion. However, it’s important to note that there are a few cases where the first electron affinity can be positive, indicating that energy is required to add an electron to the atom.