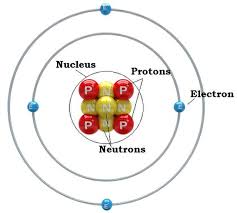
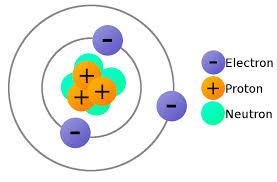
Is a Neutron Positive or Negative Charge? Atoms are made up of three components – electrons, protons and neutrons. In an atomic shell, the outermost region is occupied by electrons and the innermost region is occupied by protons and neutrons.
Electrons have a negative charge, but neutrons have no charge and have a mass 1,839 times the mass of the electron. Neutrons and protons together form the nucleus of an atom and are together called nucleons.
Is a Neutron Positive or Negative Charge?
The neutron is a subatomic particle found in the nucleus of an atom, alongside protons. Despite the presence of protons, the neutron itself is electrically neutral, meaning it has no net electrical charge. Unlike protons, which carry a positive charge, and electrons, which carry a negative charge, the neutron’s charge is zero. This characteristic is crucial for maintaining the stability of atomic nuclei. The discovery of the neutron by Sir James Chadwick in 1932 was a significant advancement in understanding atomic structure, further solidifying the understanding that atoms are composed of protons, neutrons, and electrons.
The neutral charge of the neutron results from the balance between the positively charged protons and negatively charged electrons within an atom. Protons and electrons are fundamental particles carrying opposite electrical charges but equal magnitudes. Since the neutron has no net charge, it does not experience the electromagnetic forces that affect charged particles, allowing it to reside alongside protons in the atomic nucleus without repelling or attracting them. This delicate equilibrium is vital for the stability of matter and the existence of atoms as we know them.
In summary, the neutron carries no charge, making it unique among the three fundamental particles of an atom. Its neutral nature plays a pivotal role in maintaining atomic stability and enabling the formation of elements through nuclear reactions. Learn more about Neutron here:- What are The Neutron Symbol Mass and Charge.
Why the Neutron Has no charge?
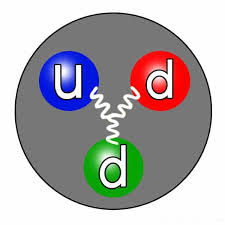
The absence of a charge in the neutron can explained by its internal composition. Neutrons consist of three fundamental particles called quarks, specifically two down quarks and one up quark. Quarks are elementary particles that carry fractional electric charges, with the up quark having a positive charge of +2/3 and the down quark having a negative charge of -1/3. When combined in a specific arrangement within the neutron, the charges of the quarks effectively cancel each other out, resulting in a net charge of zero for the neutron.
The strong nuclear force, one of the fundamental forces of nature, plays a crucial role in keeping the quarks bound together within the neutron. This force is far stronger than the electromagnetic force, which governs the interactions between charged particles. As a result, the quarks tightly confined, making it extremely difficult for them to escape and contribute to an overall electric charge for the neutron.
The concept of color charge, a property associated with quarks, also comes into play. This “color” is not related to the visual perception of color but is a quantum property that helps explain how quarks interact through the strong force. Within protons and neutrons, quarks exhibit a color-neutral configuration, ensuring their overall neutrality concerning the strong force.
In conclusion, the neutron’s lack of charge arises from the intricate balance between the electric charges of its constituent quarks and the dominance of the strong nuclear force that binds them together. This unique combination of factors renders the neutron electrically neutral and an essential component in the structure and stability of atomic nuclei.
Atomic Structure Neutron Charge
In the realm of atomic structure, the neutron’s charge, or rather, its lack thereof, is a defining characteristic. It is one of the three primary particles that constitute an atom, alongside protons and electrons. While protons bear a positive charge and electrons carry a negative charge, neutrons have a neutral charge, symbolized as 0 or “n.” The neutron’s mass is slightly greater than that of a proton, making it an essential contributor to an atom’s overall mass.
Understanding the neutron’s charge is fundamental to comprehending the behavior of atoms and their interactions with other particles. Atomic nuclei, composed of protons and neutrons, held together by the strong nuclear force, which overcomes the electrostatic repulsion between positively charged protons. If neutrons possessed a charge, this delicate balance would disrupted, leading to unstable nuclei and a significantly different universe. The neutral charge of the neutron is thus a critical factor in shaping the matter around us.
In the context of nuclear reactions, such as those occurring in nuclear power plants or during natural radioactive decay, neutrons play a pivotal role. Neutrons can released during nuclear fission, and their subsequent absorption by other nuclei can induce further fission events or trigger various processes, releasing large amounts of energy. This property harnessed in nuclear power plants for electricity generation and also utilized in nuclear weapons.
So Is a Neutron Positive or Negative Charge? In conclusion, the neutron’s charge, or lack thereof, is a fundamental aspect of atomic structure that significantly impacts the behavior and stability of matter. Its neutral nature allows it to coexist harmoniously within the atomic nucleus, ensuring the integrity of atoms and enabling various nuclear processes with far-reaching implications for energy production, medicine, and our understanding of the universe.