The Chemical properties of Potassium are as follows;
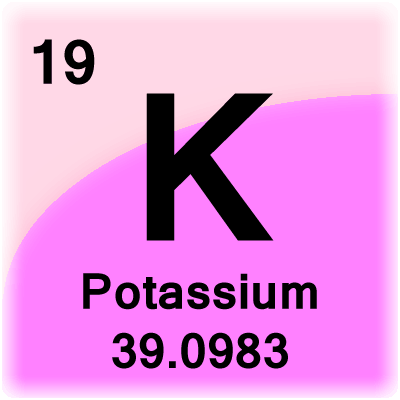
- Atomic Number- 19
- Atomic Mass – 39.0983 g.mol -1
- Electronegativity according to Pauling – 0.8
- Density – 0.86 g.cm -3 at 0 °C
- Melting point – 63.2 °C
- Boiling point – 760 °C
- Vanderwaals radius – 0.235 nm
- Ionic radius – 0.133 (+1)
- Isotopes – 5
- Electronic shell -[ Ar ] 4s1
- Ionisation energy- 418.6 kJ.mol -1
- Discovered by Sir Davy in 1808
Chemical Properties of Potassium

Potassium Periodic Table Symbol
The name of Potassium is derived from the English word potash. The chemical symbol of potassium is K. The symbol is taken from calcium that is the Mediaeval Latin word for potash, which was initially derived from the Arabic word qali that means alkali. It is a soft, white-silvery metal.
It is a member of the alkali group of the periodic table. It oxidizes instantly in the air and tarnishes within a few minutes that is why it is generally stored under grease or oil. Potassium is light so it can float in water and reacts instantly and releases hydrogen.
Who Discovered Potassium & Where is Potassium Found
In 1808, Sir Davy discovered the Potassium. It is available in the nineteenth column of the periodic table.
Facts About Potassium
- K is the seventh most abundant element on Earth.
- The Potassium was first discovered by Sir Humphry Davy in 1808.
- Potassium find through electrolysis.
- Potassium makes up 1.5% by mass of the Earth’s crust.
- It is not available as a pure element.
- Potassium (K) is available in some minerals and ionic salts.
- Electrolysis of hydroxide and chloride makes the potassium.
- After lithium, it is the second dense metal.
- It can very easily sliced.
- Argon along with other oils uses to store pure potassium.
- In water, it reacts with the hydrogen and forms heat.
What Are The Uses of Potassium?
The uses of potassium are as follows;
- It has strong properties of the base, therefore, Potassium can use to neutralize acids
- Potassium cyanide can dissolve precious metals like silver and gold.
- It can use for manufacturing daily use items.
- Potassium can used as a medium of heat transfer in many industries.
Leave a Reply