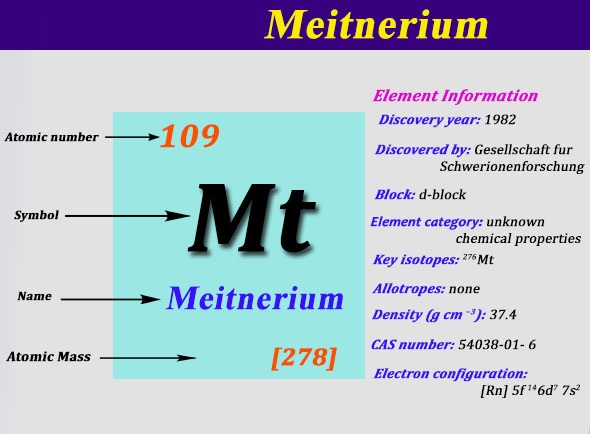
Electron Configuration For Meitnerium: Meitnerium is a synthetic chemical element that has a symbol Mt. The atomic number of Meitnerium is 109. It is a very radioactive synthetic element. The most stable known isotope of meitnerium is meitnerium-278. It has a half-life of 7.6 seconds.
However, it is unconfirmed meitnerium-282 may sometimes have a longer half-life of 67 seconds. Meitnerium is ad-block transactinide element In the periodic table.
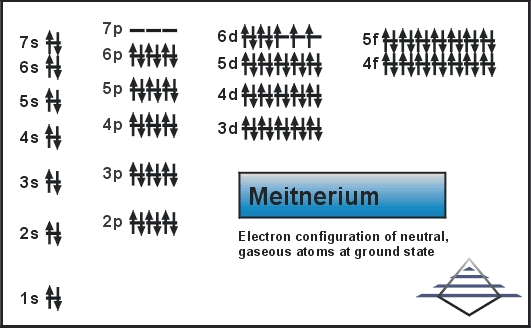
Meitnerium is calculated to have similar characteristics to its lighter homologues, rhodium, cobalt, and iridium. Today we are going to share the information about the electron configuration of the Mt. If you are also here to get the information of the same element then you are at the right place. Please read the full post below.
What is the Electron Configuration of Meitnerium
[Rn] 5f14 6d7 7s2 is the electron configuration of the Meitnerium.
How Many Valence Electrons Does Meitnerium Have
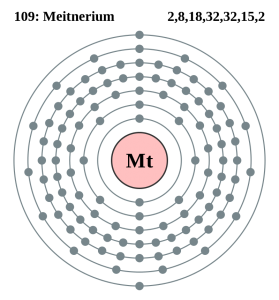
There are twenty-three valence electrons in the outer shell of the Meitnerium.
Meitnerium Number of Valence Electrons
Meitnerium has twenty-three valence electrons in its outer shell.
Leave a Reply