Learn about the Manganese electron configuration here and make the systematic learning of the chemical element. The article provides thorough knowledge on the electron configuration of the Manganese so that they can understand the element in a good manner.
Manganese is basically a chemical element in the chemistry branch of science. It has the atomic number 25 and the represents symbol of mn. In its physical structure, the chemical element looks like the hard silvery metal with a brittle outlook. The chemical element is easily available in the minerals with the composition of iron.
Manganese Electron Configuration
Furthermore, Manganese is a highly used and scalable chemical element that has a number of industrial usages. For instance, you can see the Manganese application in industrial usage such as in the production of alloys, stainless steel, etc. This is what makes the Manganese one of the highly popular and useful chemical elements in the industrial domain. The element is easily available in a significant amount across the globe and serves significant practical purposes in the industrial domain.
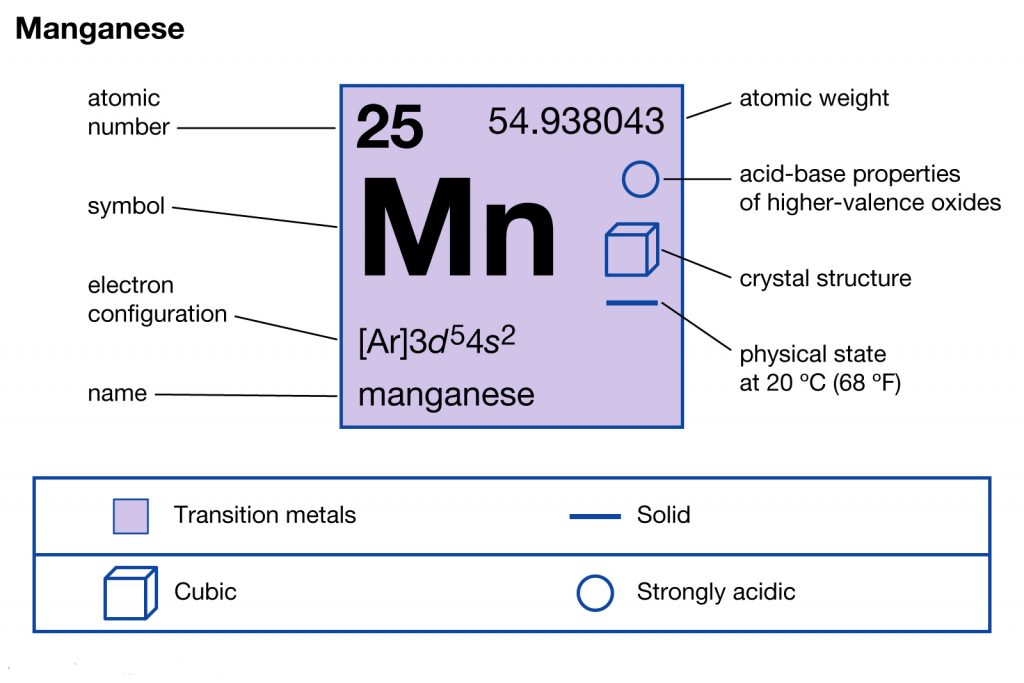
Well, the Mn electron configuration is significant for all the scholars who are studying the element. The electron configuration of the Manganese element basically states the procedure of distributing the electrons to the atomic orbitals. This whole process or the equation of the electron distribution is what becomes the electron configuration.
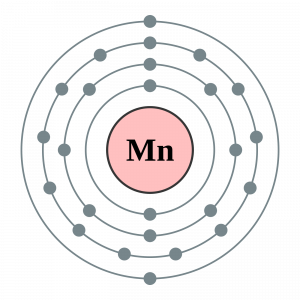
So, the Manganese electron configuration can basically be written as the 1s22s22p63s23p64s23d5 for understanding purposes. The electron configuration is the significant chemical properties of the chemical element.
How many valence electrons does Manganese have?
Furthermore, the electron configuration of the Manganese closely associates itself with the atomic number of the chemical element. It helps in the overall simplification of the chemical element for a depth understanding of the element. So, for the same reason, the electron configuration of Mn becomes a highly useful topic to explore and understand in an appropriate manner.
Electron Configuration for Mn
Well, if you are wondering about the derivation of the Mn electron configuration then you are at the correct place. Here in this section, we are going to simplify the whole derivation concept for the electron configuration of Manganese. You can easily understand the derivation concept in easy step-by-step formats.
- With the Aufbau principle, the first orbital has 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on electrons.
- Subsequently, the s orbital will contain 2 electrons, the p orbital will contain 2 electrons and then the s orbital will have the 10 electrons at max.
- At last, we will have the following equation of the electrons configuration for Manganese as 1s22s22p63s23p64s23d5.
So, this is how we will get our final equation for the Mn electron configuration. We believe this derivation technique would make it easier for you to understand the Manganese electron configuration. Feel free to share it with the other scholars of chemistry.
Leave a Reply