We are here providing the Lead valence electrons to all our readers. They can further explore the other information of the element. In chemistry, lead is a chemical element with its symbol of Pb. Lead is one of the densest and heavy chemical elements.
How many valence electrons does Lead have?
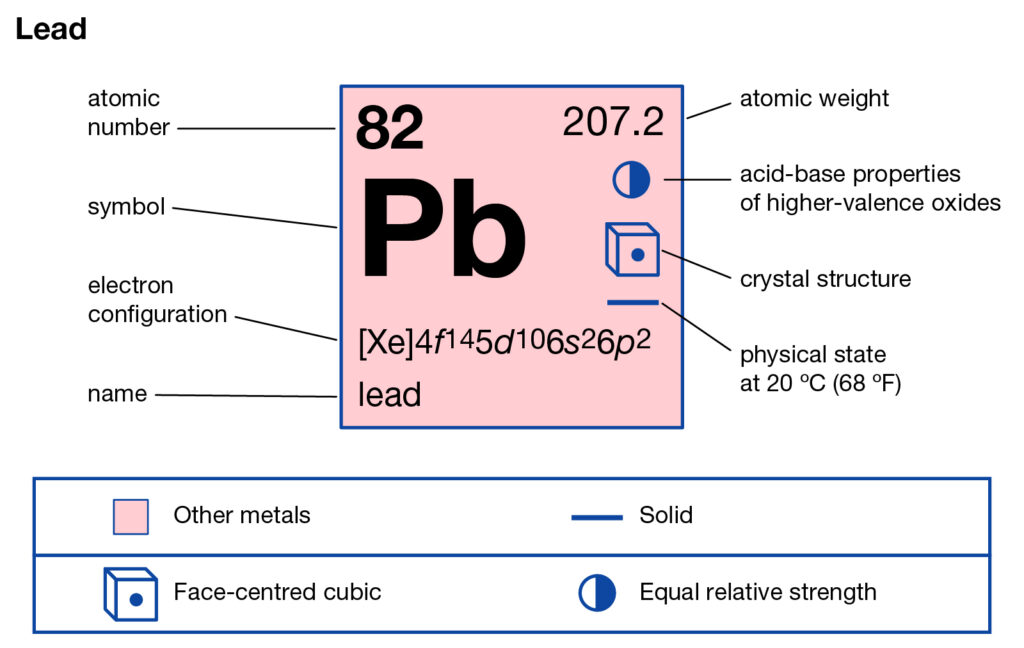
It further has an atomic number of 82 which is the highest for any possible stable element. The lead looks like a silvery soft metal but turns into dull grayish in the exposure of air. Lead is although a transition metal, however, it has very little to no reactive properties. It is easily available in its natural occurrence ores mines. Check out the position of Pb on the periodic table.
Lead occurs from the ores along with the silver. In ancient times the extraction of silver gave a huge boost to lead extraction as well. The major use of leads goes to the production of bullets. Leads have a very significant contribution to the production of ammunition.
Further lead is useful as an addition to the other metals. For instance, it is useful to use along with copper in creating the various types of sculptures. The high density, atomic number of leads makes it the superior barrier of sound and vibration. It is one such strong metal that can withhold the major storms.
Lead Valence Electrons Dot Diagram
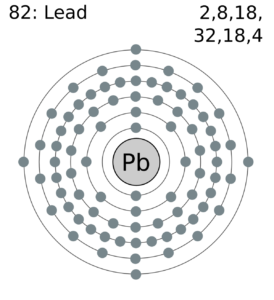
Feel free to understand the lead valence electrons with our lead dot diagram. You can use the diagram to understand the chemical binding of valence electrons.
The diagram depicts the numbers of valence electrons of atoms around the symbol of lead. You can understand the interaction of valence electrons with this dot diagram in a better way.
Valency of Lead
Lead Valence Electrons
-
Element symbol: Pb
-
Atomic number: 82
-
Electron configuration: [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p²
-
Valence electrons: 4
-
Located in: Group 14 (IVA) of the periodic table
-
Valence shell: 6th shell → 6s² 6p²
-
Common oxidation states: +2 and +4
Lead has two valencies which may be either of 2 or 4. It is the transition chemical element with a high atomic value that has variable valency.
Leave a Reply