The periodic table is a fundamental tool in chemistry, organizing elements based on their atomic number and chemical properties. It serves as a reference guide, allowing scientists to understand the relationships and patterns among different elements. However, have you ever wondered How Many Periods Are In The Periodic Table? In this article, we will explore the structure of the periodic table, the concept of periods, and how they contribute to our understanding of chemical elements.
How Many Periods Are In The Periodic Table
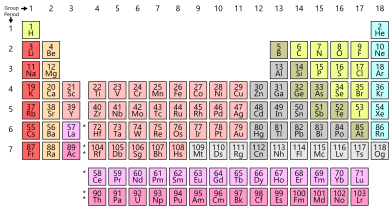
The periodic table is organized into rows and columns, which provide valuable information about the properties of elements. These rows are known as “periods,” and they play a fundamental role in the table’s structure. As of my knowledge cutoff in September 2021, the periodic table consists of seven periods.
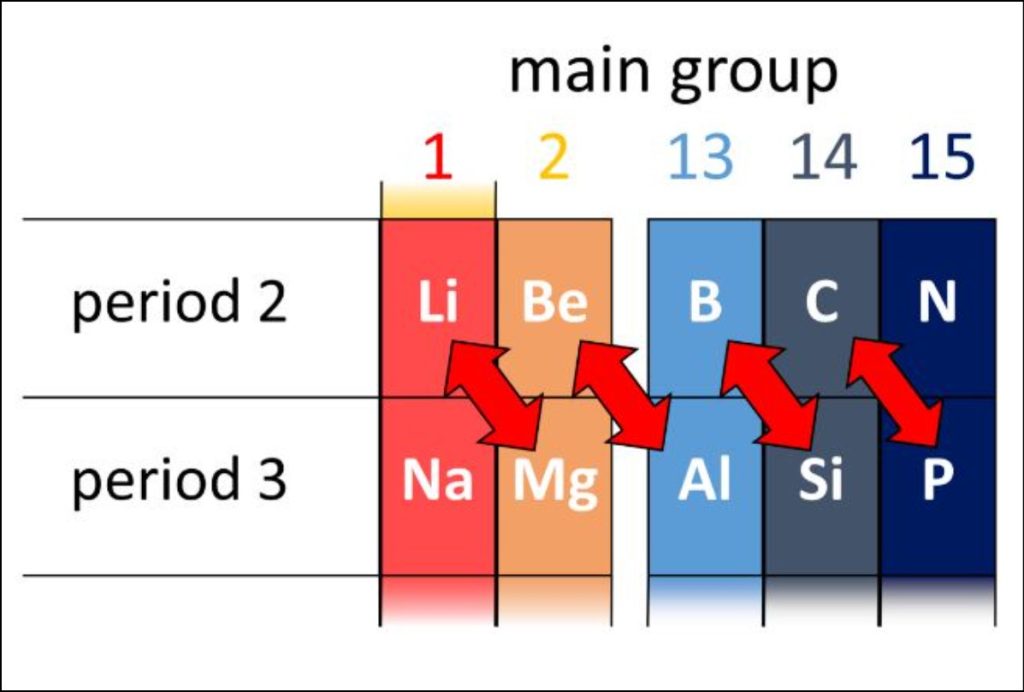
Each period represents a principal energy level and corresponds to the number of electron shells an atom possesses. Period 1 contains only two elements – hydrogen and helium – as they have the simplest atomic structures. As we move down the periodic table, the number of elements within each period increases, with period 7 having the highest number of elements.
Within each period, the elements’ properties change in a predictable manner. As we move from left to right across a period, the atomic number and atomic mass increase, and the properties of the elements become progressively less metallic and more non-metallic. Furthermore, the period number directly indicates the highest energy level or shell that the electrons of the elements in that period occupy.
It’s important to note that the periodic table can expanded beyond the current seven periods if more elements are discovered beyond the current heaviest elements known.
Periodic Table Groups and Periods
The periodic table is organized into groups and periods, which are crucial for understanding the chemical behavior and properties of elements. Elements within the same group exhibit similar chemical properties, as they have the same number of valence electrons, while elements within the same period share similar electron configurations and atomic energy levels.
Groups, also known as families, are the columns present in the periodic table, and numbered from 1 to 18. The elements within each group share the same number of valence electrons, making them behave similarly in chemical reactions. For instance, Group 1 elements, known as alkali metals, all have one valence electron and are highly reactive metals. Group 17 elements, or halogens, have seven valence electrons and are known for their reactivity with metals to form salts.
Periods, on the other hand, are the rows in the periodic table, numbered from 1 to 7. Each period represents one principal energy level or shell of the elements. Elements within the same period have the same highest energy level occupied by their electrons. For example, all elements in period 3 have their outermost electrons in the third energy level.
Understanding the arrangement of groups and periods in the periodic table is essential for predicting and explaining the chemical properties and reactivity of different elements.
What Do Group And Period Mean in the Periodic Table
In the context of the periodic table, “group” and “period” are two key concepts that organize the elements based on their electronic configurations and properties.
A “group” in the periodic table refers to the vertical columns of elements. Elements within the same group have similar chemical properties because they share the same number of valence electrons. Valence electrons, the outermost electrons involved in chemical reactions, and they largely determine an element’s reactivity and bonding behavior. For instance, elements in Group 1 (e.g., lithium, sodium, potassium) all have one valence electron, which makes them highly reactive and prone to forming +1 ions. Similarly, elements in Group 18 (e.g., helium, neon, argon) have full valence electron shells and are chemically inert.
A “period” in the periodic table refers to the horizontal rows of elements. Elements within the same period have their electrons in the same principal energy level or electron shell. As we move from left to right across a period, the atomic number increases sequentially, and the elements’ properties change in a predictable manner. This trend is due to the progressive filling of electron shells and the change in the effective nuclear charge experienced by the outer electrons.
In summary, groups and periods in the periodic table help us understand the relationships between the elements and predict their chemical behavior based on their electron configurations.
Difference Between a Group And a Period on the Periodic Table
The periodic table structured into groups and periods, and each serves a distinct purpose, offering valuable information about the elements.
A “group” in the periodic table represents the vertical columns, while a “period” represents the horizontal rows. The main difference between the two lies in the properties they describe. Groups primarily indicate the number of valence electrons an element possesses. Elements within the same group share similar chemical properties due to having the same number of valence electrons. This similarity in valence electron configuration leads to comparable chemical reactivity and bonding behavior among elements in the same group.
On the other hand, periods indicate the principal energy level or electron shell in which the elements’ valence electrons are located. As we move from left to right across a period, the atomic number increases, meaning more protons and electrons are present in the atoms. This results in a gradual change in the elements’ properties as we move across a period. Elements within the same period do not necessarily exhibit similar chemical properties, as the number of valence electrons may differ significantly from one element to another.
In summary, groups associated with similar chemical properties based on the number of valence electrons, while periods are related to the highest energy level or electron shell occupied by the elements’ valence electrons. Understanding both groups and periods is essential for grasping the periodic table’s patterns and predicting the chemical behavior of different elements.
Leave a Reply