Do you want to learn the Europium election configuration for the next chemistry class? Well, you are here at the correct place for the same cause. Here in the article, you will find the proper information on the Europium electron configuration and the other properties of the element.
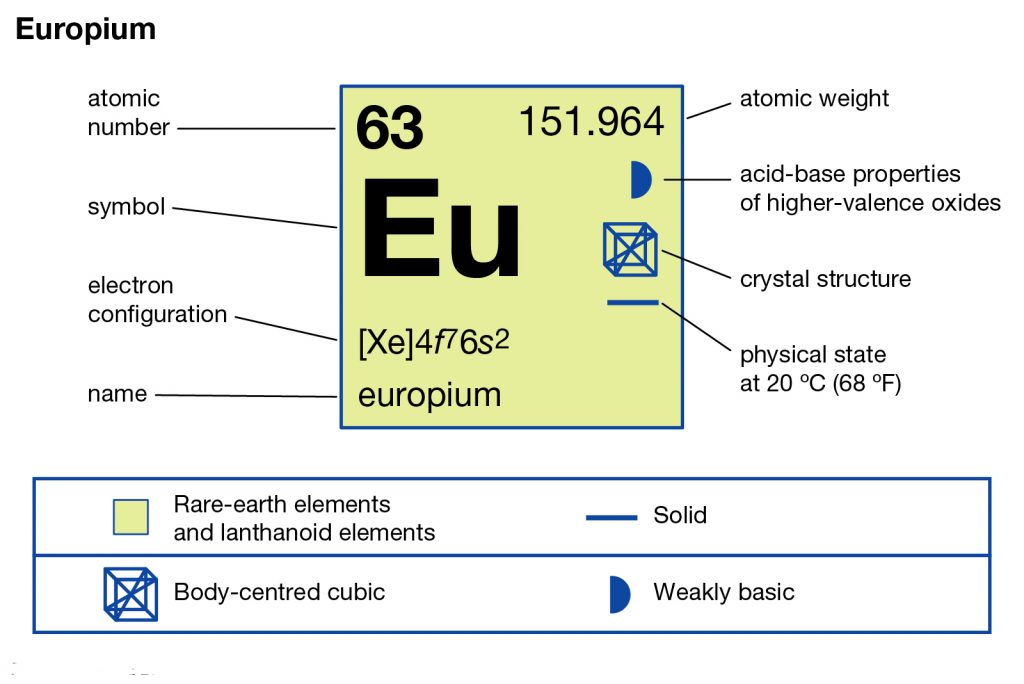
Europium is a well-recognized chemical element in the domain of chemistry that falls in the category of Lanthanide elements. It has the atomic number 63 and the symbolic sign of Eu in the periodic table of chemistry. Europium has the tag as the most reactive chemical element that belongs to the family of Lanthanide elements. For the same reason, the chemical element generally has very safe storage that is free from exposure to oxygen or moisture.
Europium Electron Configuration
Furthermore, the other specialty of the Europium is its softer side since the element is so soft that you can twist it with mere fingers. The element was first discovered in 1901 within Europe and for the same, it has a similar name. Since Europium is a member of the Lanthanide family therefore it has all the properties of the same category. For instance, the element can easily assume the oxidation states either +2 or +3 quickly. Europium is one of the rarest chemical elements in the world with a very limited supply.
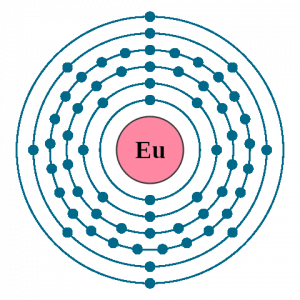
Well, the chemical element Europium is incomplete from its exploration unless you study its electron configuration. The electron configuration is basically the process by which Europium distributes its electrons to its own orbitals. This whole distribution is what becomes the electron configuration of the Europium element. This process is mandatory for each and every chemical element in the periodic table of chemistry.
Electron Configuration for Eu
So, the electron configuration of Europium is Xe 4f7 6s2 in its short abbreviated form which is also written in the periodic table. The electron configuration equation is necessary for the overall breakout of the chemical element. With the Europium electron configuration scientist or chemists can find the other chemical properties of the element. Subsequently, they can end up finding the other several applications of the element which are not in the knowledge presently. We urge you to read the chemical element in its periodic table to find its other chemical properties.
How many valence electrons does Europium have?
Europium is still in its early phase in the context of its commercial application in the practical world. The chemical element is extremely expensive and for the same reason, it has very specialized usage. The chemical element is further rare in its availability as it’s not easily available everywhere in the world. You can find the standard application of Europium as the dopant in the number of lasers and the several types of glasses.
Some part of the chemical element is also useful to be used as the phosphor in the television sets. The screens of the TV contain some amount of Europium dioxide due to which the screen is one of the most expensive components of the television. The chemical element is although quite safe even in human exposure. So, this is all about the Europium electron configuration and the other properties of this element. Do share the article with others as well who want to study the Europium for their learning.
Leave a Reply