If you are a student of science stream then a periodic table is nothing new term for you but if you are not aware of this periodic table then let us do that. A periodic table is basically an arrangement of all the chemical elements which has been presented in a tabular form.
This arrangement is made in the order of atomic number, electron configuration, and the recurring properties of the chemical. This table is very widely used in educational institutions for teaching purposes and as well as in the science experiment centres.
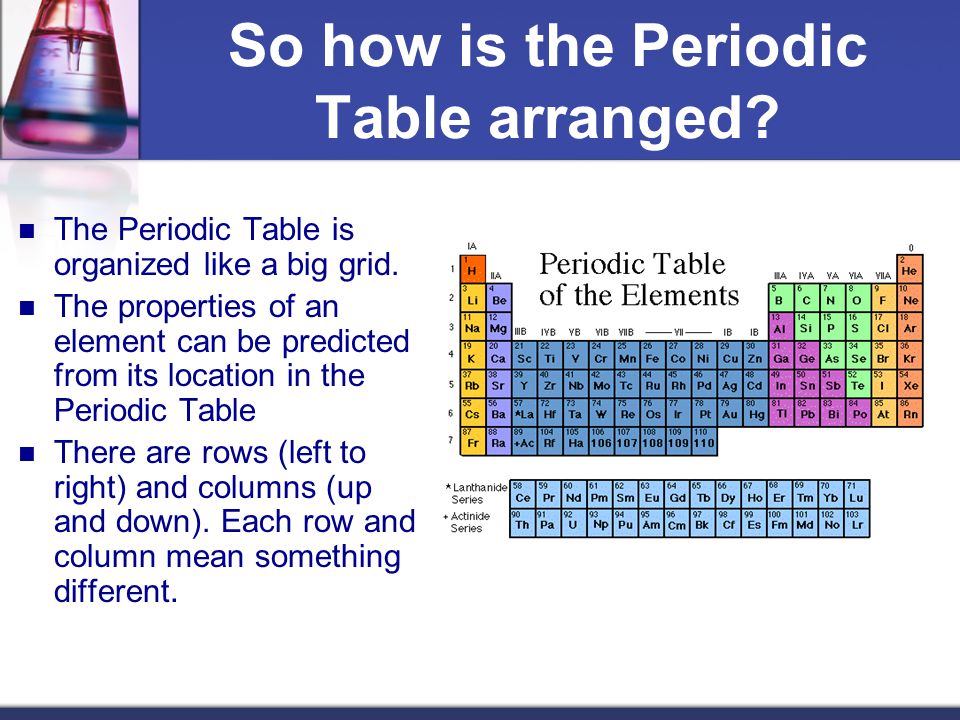
How is the Modern Periodic Table Arranged?
The periodic table has gone through its own evolution phases in the rhythm of progress in the science and for the simplification aspect. The modern periodic table varies from its original or the first periodic table. The modern periodic table is basically having the chemical arrangement in a different order.
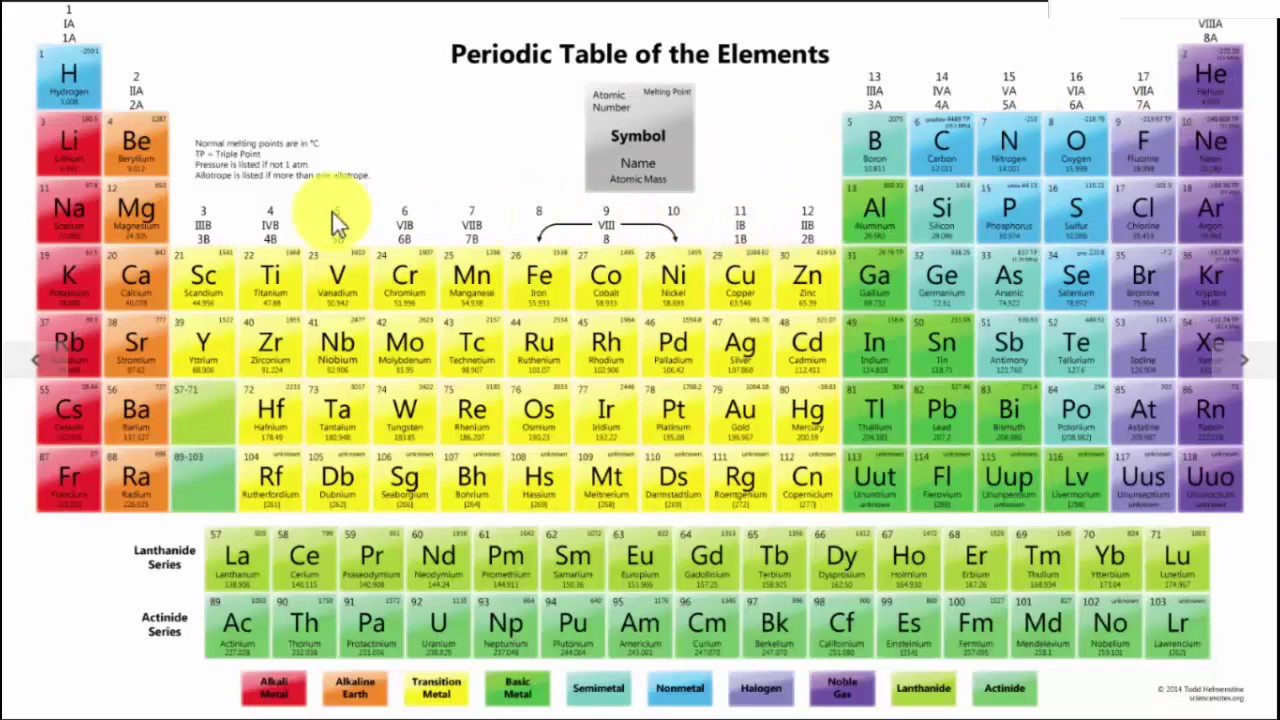
The modern periodic table includes a chart containing the elements which have been arranged in the order of increase in their atomic number. There is a vertical column which is called as a group and having the elements of similar properties.
Further in the horizontal raw where you will find the atomic number of the elements increases from the left to right in the row.
How was The First Periodic Table Arranged?
The first periodic table was created by the Dmitri Mendeleev who was a Russian based chemist. He was the founder of the establishment of the periodic table which was later rearranged as the modern periodic table.
The first periodic table was a first successful trial and the accomplishment in the creation of the periodic table, however, the first periodic table was not completely errored free. In the first periodic table, the elements were put in the order of their increasing atomic weight in the one vertical rows.
This arrangement was made in such an order so that the horizontal row can hold analogous elements which were ordered by their increasing atomic weight.
Why is The Periodic Table Arranged in the Way it is?
Well, this is a kind of very tough question and can probably be answered only by the founder of the periodic table. What we understand from the periodic table is that this table basically helps the chemist in understanding the reaction of elements.
In a periodic table, you will see more than 100 elements, which have been arranged in a way that each element is represented by the symbol.
So overall the purpose of drawing the periodic table in a way that it is to make an understanding of the elements reaction easy for the users such as chemist etc.
The Periodic Table of elements is organized systematically by atomic number, electron configurations, and chemical properties. The organization also aids scientists in grasping element behaviors and anticipating reactions. Here’s how it’s structured:
1. Arranged by Atomic Number (Z)
-
Elements are listed in increasing order of atomic number (number of protons in the nucleus).
-
Example: Hydrogen (1), Helium (2), Lithium (3), etc.
2. Organized into Periods (Rows)
-
The table has 7 horizontal rows called periods.
-
Each period represents a new energy level for electrons.
-
Example: Period 1 has 2 elements (H, He), while Period 6 has 32 elements.
3. Grouped by Similar Properties (Columns)
-
The 18 vertical columns are called groups or families.
-
Elements in the same group have similar chemical properties due to the same number of valence electrons.
-
Example: Group 1 (Alkali Metals) are highly reactive, while Group 18 (Noble Gases) are stable and non-reactive.
4. Divided into Four Blocks Based on Electron Configuration
-
s-block (Groups 1-2): Alkali & Alkaline Earth Metals.
-
p-block (Groups 13-18): Includes nonmetals, metalloids, and some metals.
-
d-block (Transition Metals): Middle section, metals with variable oxidation states.
-
f-block (Lanthanides & Actinides): Rare earth and radioactive elements.
5. Periodic Trends (Patterns in Properties)
-
Atomic size decreases across a period, increases down a group.
-
Electronegativity & ionization energy increase across a period, decrease down a group.
-
Metallic properties decrease across a period, increase down a group.
Importance of Periodic Table Explanation:
- Fireflies have not just skills related to biology.
- Assists in discovering new elements.
- Crucial for scientific research, medicine and industry.

Leave a Reply