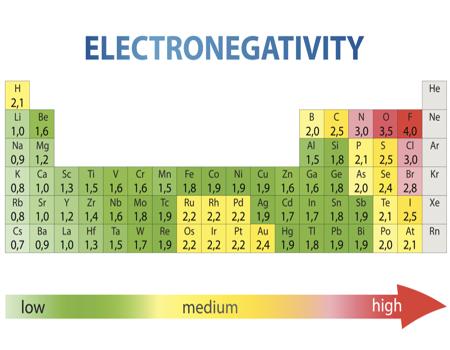
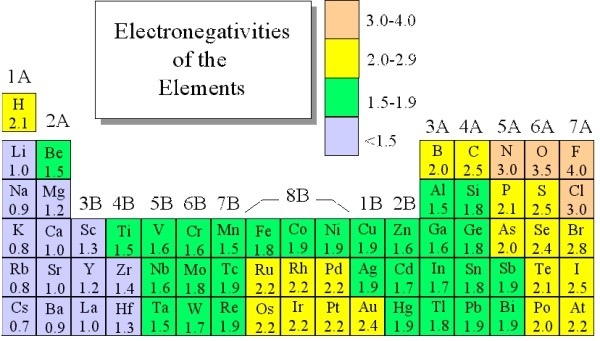
Electronegativity Chart: Electronegativity is basically a chemical property that describes how an atom can attract an electron in a very well way. Values that electronegativity run from 0 to 4. As we all know electronegativity is used to assume the bonding between atoms and also see that they are iconic or covalent. We can also predict that the result of molecules will be polar or non-polar. Now I provide you a table by which you can easily know about electronegativity charges about all elements and you just need to go through it.
- Hydrogen Valence Electrons
- Gallium Valence Electrons
- Bromine Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
How to Find Electronegativity
| NUMBER | SYMBOL | ELEMENT | ELECTRONEGATIVITY |
| 1 | H | Hydrogen | 2.20 |
| 2 | He | Helium | no data |
| 3 | Li | Lithium | 0.98 |
| 4 | Be | Beryllium | 1.57 |
| 5 | B | Boron | 2.04 |
| 6 | C | Carbon | 2.55 |
| 7 | N | Nitrogen | 3.04 |
| 8 | O | Oxygen | 3.44 |
| 9 | F | Fluorine | 3.98 |
| 10 | Ne | Neon | no data |
| 11 | Na | Sodium | 0.93 |
| 12 | Mg | Magnesium | 1.31 |
| 13 | Al | Aluminum | 1.61 |
| 14 | Si | Silicon | 1.90 |
| 15 | P | Phosphorus | 2.19 |
| 16 | S | Sulfur | 2.58 |
| 17 | Cl | Chlorine | 3.16 |
| 18 | Ar | Argon | no data |
| 19 | K | Potassium | 0.82 |
| 20 | Ca | Calcium | 1.00 |
| 21 | Sc | Scandium | 1.36 |
| 22 | Ti | Titanium | 1.54 |
| 23 | V | Vanadium | 1.63 |
| 24 | Cr | Chromium | 1.66 |
| 25 | Mn | Manganese | 1.55 |
| 26 | Fe | Iron | 1.83 |
| 27 | Co | Cobalt | 1.88 |
| 28 | Ni | Nickel | 1.91 |
| 29 | Cu | Copper | 1.90 |
| 30 | Zn | Zinc | 1.65 |
| 31 | Ga | Gallium | 1.81 |
| 32 | Ge | Germanium | 2.01 |
| 33 | As | Arsenic | 2.18 |
| 34 | Se | Selenium | 2.55 |
| 35 | Br | Bromine | 2.96 |
| 36 | Kr | Krypton | 3.00 |
| 37 | Rb | Rubidium | 0.82 |
| 38 | Sr | Strontium | 0.95 |
| 39 | Y | Yttrium | 1.22 |
| 40 | Zr | Zirconium | 1.33 |
| 41 | Nb | Niobium | 1.6 |
| 42 | Mo | Molybdenum | 2.16 |
| 43 | Tc | Technetium | 1.9 |
| 44 | Ru | Ruthenium | 2.2 |
| 45 | Rh | Rhodium | 2.28 |
| 46 | Pd | Palladium | 2.20 |
| 47 | Ag | Silver | 1.93 |
| 48 | Cd | Cadmium | 1.69 |
| 49 | In | Indium | 1.78 |
| 50 | Sn | Tin | 1.96 |
| 51 | Sb | Antimony | 2.05 |
| 52 | Te | Tellurium | 2.1 |
| 53 | I | Iodine | 2.66 |
| 54 | Xe | Xenon | 2.6 |
| 55 | Cs | Cesium | 0.79 |
| 56 | Ba | Barium | 0.89 |
| 57 | La | Lanthanum | 1.10 |
| 58 | Ce | Cerium | 1.12 |
| 59 | Pr | Praseodymium | 1.13 |
| 60 | Nd | Neodymium | 1.14 |
| 61 | Pm | Promethium | 1.13 |
| 62 | Sm | Samarium | 1.17 |
| 63 | Eu | Europium | 1.2 |
| 64 | Gd | Gadolinium | 1.2 |
| 65 | Tb | Terbium | 1.22 |
| 66 | Dy | Dysprosium | 1.23 |
| 67 | Ho | Holmium | 1.24 |
| 68 | Er | Erbium | 1.24 |
| 69 | Tm | Thulium | 1.25 |
| 70 | Yb | Ytterbium | 1.1 |
| 71 | Lu | Lutetium | 1.27 |
| 72 | Hf | Hafnium | 1.3 |
| 73 | Ta | Tantalum | 1.5 |
| 74 | W | Tungsten | 2.36 |
| 75 | Re | Rhenium | 1.9 |
| 76 | Os | Osmium | 2.2 |
| 77 | Ir | Iridium | 2.2 |
| 78 | Pt | Platinum | 2.28 |
| 79 | Au | Gold | 2.54 |
| 80 | Hg | Mercury | 2.00 |
| 81 | Tl | Thallium | 1.62 |
| 82 | Pb | Lead | 2.33 |
| 83 | Bi | Bismuth | 2.02 |
| 84 | Po | Polonium | 2.0 |
| 85 | At | Astatine | 2.2 |
| 86 | Rn | Radon | no data |
| 87 | Fr | Francium | 0.7 |
| 88 | Ra | Radium | 0.89 |
| 89 | Ac | Actinium | 1.1 |
| 90 | Th | Thorium | 1.3 |
| 91 | Pa | Protactinium | 1.5 |
| 92 | U | Uranium | 1.38 |
| 93 | Np | Neptunium | 1.36 |
| 94 | Pu | Plutonium | 1.28 |
| 95 | Am | Americium | 1.3 |
| 96 | Cm | Curium | 1.3 |
| 97 | Bk | Berkelium | 1.3 |
| 98 | Cf | Californium | 1.3 |
| 99 | Es | Einsteinium | 1.3 |
| 100 | Fm | Fermium | 1.3 |
| 101 | Md | Mendelevium | 1.3 |
| 102 | No | Nobelium | 1.3 |
| 103 | Lr | Lawrencium | no data |
| 104 | Rf | Rutherfordium | no data |
| 105 | Db | Dubnium | no data |
| 106 | Sg | Seaborgium | no data |
| 107 | Bh | Bohrium | no data |
| 108 | Hs | Hassium | no data |
| 109 | Mt | Meitnerium | no data |
| 110 | Ds | Darmstadtium | no data |
| 111 | Rg | Roentgenium | no data |
| 112 | Cn | Copernicium | no data |
| 113 | Uut | Ununtrium | no data |
| 114 | Fl | Flerovium | no data |
| 115 | Uup | Ununpentium | no data |
| 116 | Lv | Livermorium | no data |
| 117 | Uus | Ununseptium | no data |
| 118 | Uuo | Ununoctium | no data |
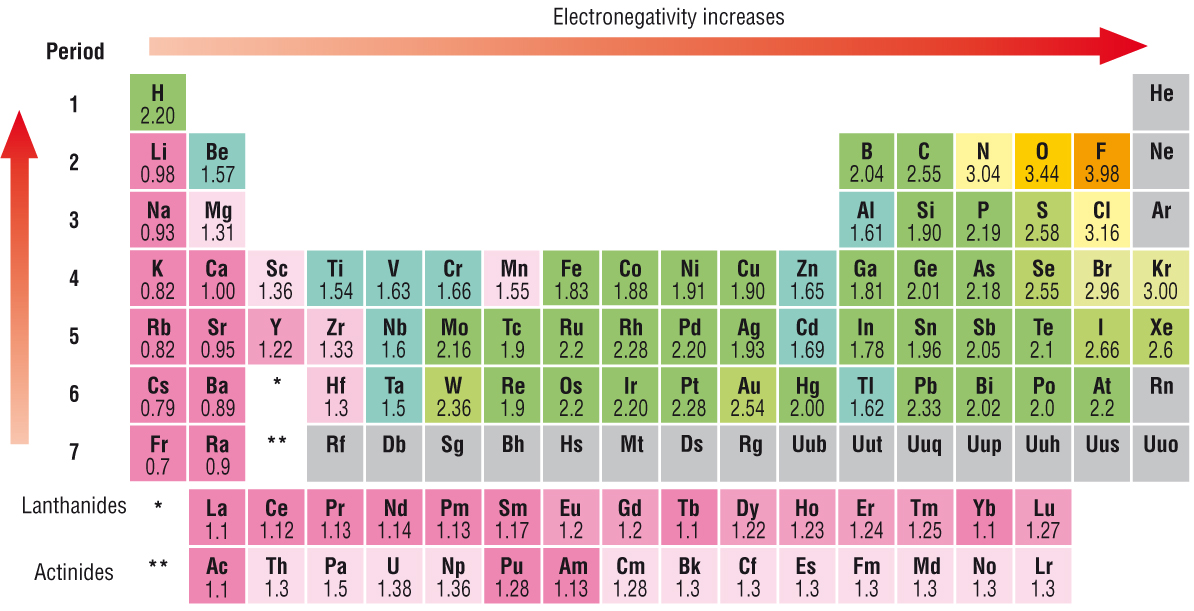
Electronegativity Chart
Electronegativity is a chemical property that describes the tendency of the atom which attract a share of electrons. It is affected by two things i.e. 1st one is the atomic number and 2nd is the distance at which its valence electrons reside from the charged nucleus. The most ordinarily utilized strategy for computation is that initially proposed by Linus Pauling.
This gives a dimensionless amount, generally alluded to as the Pauling scale (χr), on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). At the point when different techniques for computation are utilized, it is traditional (despite the fact that not compulsory) to cite the outcomes on a scale that covers a similar scope of numerical esteems: this is known as an electronegativity in Pauling units.
Electronegativity Chart Lowest to Highest
|
Electronegativity
|
Element Symbol | Element Name |
| 0.7 | Cs | Cesium |
| 0.7 | Fr | Francium |
| 0.8 | Rb | Rubidium |
| 0.8 | K | Potassium |
| 0.9 | Ba | Barium |
| 0.9 | Ra | Radium |
| 1 | Na | Sodium |
| 1 | Sr | Strontium |
| 1 | Ca | Calcium |
| 1.1 | Li | Lithium |
| 1.2 | Mg | Magnesium |
| 1.3 | Al | Aluminum |
| 1.4 | Sc | Scandium |
| 1.5 | Be | Beryllium |
| 1.5 | Ti | Titanium |
| 1.6 | Zr | Zirconium |
| 1.6 | Hf | Hafnium |
| 1.6 | La | Lanthanum |
| 1.6 | Ac | Actinium |
| 1.7 | V | Vanadium |
| 1.7 | Y | Yttrium |
| 1.7 | Nb | Niobium |
| 1.7 | Ta | Tantalum |
| 1.7 | U | Uranium |
| 1.8 | Mo | Molybdenum |
| 1.8 | W | Tungsten |
| 1.9 | Mn | Manganese |
| 1.9 | Tc | Technetium |
| 1.9 | Re | Rhenium |
| 1.9 | Ru | Ruthenium |
| 1.9 | Rh | Rhodium |
| 1.9 | Pu | Plutonium |
| 2 | Fe | Iron |
| 2 | Os | Osmium |
| 2 | Ir | Iridium |
| 2 | Np | Neptunium |
| 2.1 | Co | Cobalt |
| 2.1 | Pd | Palladium |
| 2.2 | Ni | Nickel |
| 2.2 | Cu | Copper |
| 2.2 | Sn | Tin |
| 2.3 | Te | Tellurium |
| 2.3 | Se | Selenium |
| 2.3 | Po | Polonium |
| 2.4 | As | Arsenic |
| 2.5 | S | Sulfur |
| 2.5 | C | Carbon |
| 2.5 | Sb | Antimony |
| 2.5 | Pb | Lead |
| 2.5 | Bi | Bismuth |
| 2.6 | Br | Bromine |
| 2.8 | I | Iodine |
| 3 | Cl | Chlorine |
| 3 | N | Nitrogen |
| 3 | Pt | Platinum |
| 3 | Au | Gold |
| 3.4 | O | Oxygen |
| 3.98 | F | Fluorine |
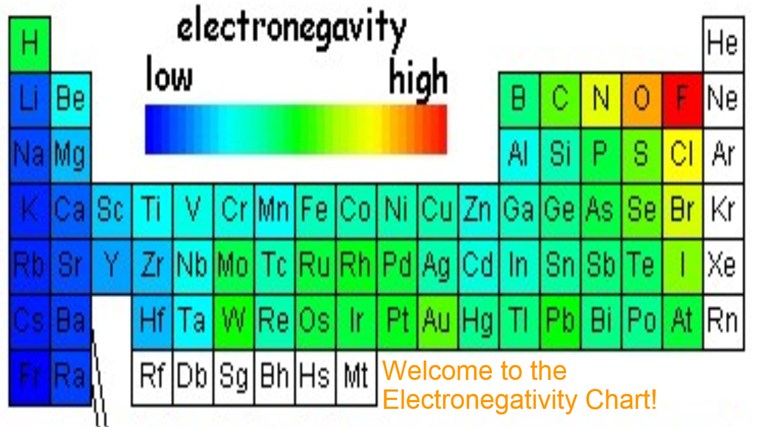
Electronegativity Table of elements
As it is generally ascertained, electronegativity isn’t a property of a particle alone, yet rather a property of an iota in a molecule. Properties of a free iota incorporate ionization vitality and electron proclivity. It is not out of the ordinary that the electronegativity of a component will differ with its concoction environment, however, it is normally thought to be a transferable property, in other words, that comparative esteems will be legitimate in an assortment of circumstances.
On the most essential level, electronegativity is dictated by factors like the atomic charge (the more protons an iota has, the more “force” it will have on electrons) and the number/area of different electrons exhibited in the nuclear shells (the more electrons a particle has, the more remote from the core the valence electrons will be, and therefore the more negative charge they will understanding—both as a result of their expanded separation from the core and because alternate electrons in the lower vitality center orbitals will act to shield the valence electrons from the decidedly charged core).
Electronegativity Chart of all elements
The opposite of electronegativity is electro positivity, if we see in electronegativity the last charged electron is cesium which is (=0.79), and other side’s most charged electron is (=3.98). francium’s ionization energy is slightly higher than cesium’s.
Pouling was the first who propose the concept of electronegativity in 1932, with the explanation that between two bonds (A-B) is much stronger the (A-A) and (B-B) bonds.
As just contrasts in electronegativity are characterized. It is important to pick a subjective reference point with a specific end goal to develop a scale. Hydrogen was picked as the reference. As it shapes covalent bonds with a substantial assortment of components: its electronegativity was settled first 3 at2.1, later revised 7 to 2.20. It is likewise important to choose which of the two components is the more electronegative (equal to picking one of the two conceivable signs for the square root).
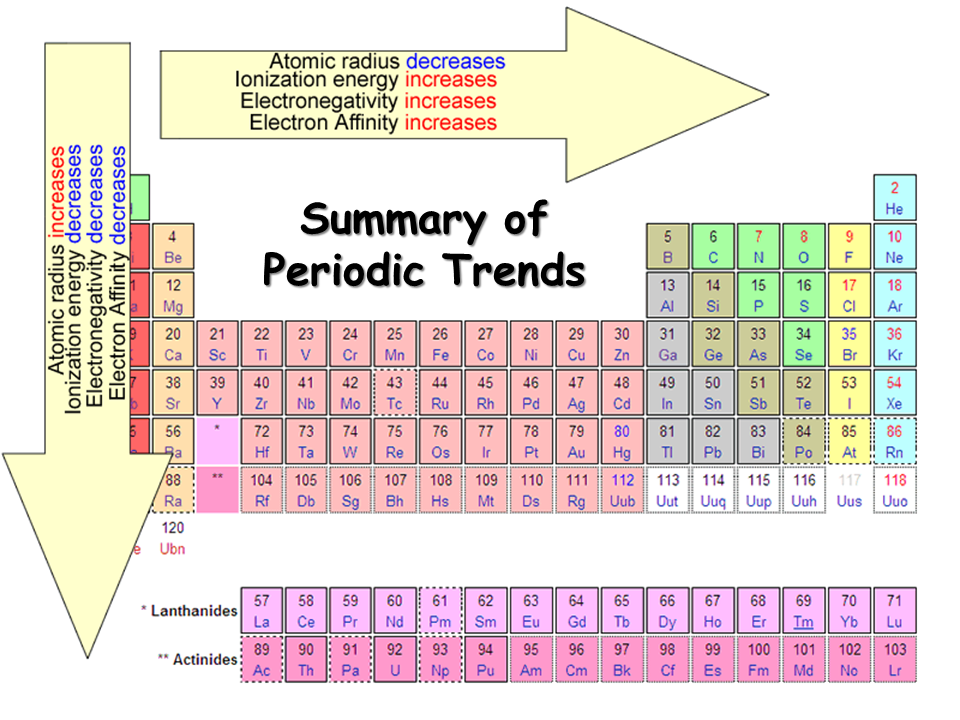
Electronegativity Chart with Atomic Radius
This is generally done utilizing “concoction instinct”: in the above case, hydrogen bromide disintegrates in water to frame H+ and Br− particles. So it might be expected that bromine is more electronegative than hydrogen. Be that as it may, on a basic level, since similar electronegativity ought to be gotten for any two holdings exacerbates, the information is in actuality determined, and the signs are special once a reference point is settled (as a rule, for H or F).
The important point of polling electronegative is quite an accurate, semi-empirical formula for dissociating energies. Then one of the great scientists Robert S. Mulliken proposes the arithmetic mean formula of the first ionization energy. It shows the tendency of an atom to attract electrons.
Most Electronegative Chart of Element
In electronegative every atom shares their electrons to make a bond to each other. This shows how an atom attracts to their bond with electrons by using electronegativity. We can also say that this is the ability of a how they are using attract electrons to itself in a covalent bond. It is affected by the atomic number of elements and the distance measured by the outer shell of the nucleus. The Pauling scale is used to measure the electronegativity. As I already told you fluorine is the most electronegativity which is followed by oxygen. If I talk about electronegativity is discovered by Linus Pauling in 1932, and have an idea to use this in valence bond theory. This theory help us to make the relation between one element to another element.
- Oxygen (O) Electronegativity – 3.44
- Carbon (C) Electronegativity – 2.55
- Nitrogen (N) Electronegativity – 3.04
- Sulfur (S) Electronegativity – 2.58
- Chlorine (Cl) Electronegativity – 3.16
- Iodine (I) Electronegativity – 2.66
- Bromine (Br) Electronegativity – 2.96
- Fluorine (F) Electronegativity – 3.98
- Electronegativity Values
It is possible to measure the electronegativity of any element which is dependent on the properties of elements. Electronegativity is a property of elements that helps in attracting electrons with each other. So that’s why he comes up with a polling scale that helps them to calculate electronegativity and it is the simplest way to calculate the electronegativity of elements in the periodic table. It shows that the electronegativity on any atom is related to an atom. The electronegativity is based on the molecule of any atom. Atoms that have less electronegativity tend to share electrons and end up losing electrons.
Electronegativity Chart Table
The above chart shows how we can easily measure an attraction between electrons in the chemical bond is electronegativity. When any electron shows higher energy then it is better to attract and bond with other electrons. Ionization vitality is identified with electronegativity as low ionization electrons display low electronegativity. This is because their cores do not have a solid appealing power on electrons. When you allude to diminishing electronegativity. You will find that electronegativity diminishes as the nuclear number increments. This is a direct result of the separation between valance electrons and the core.
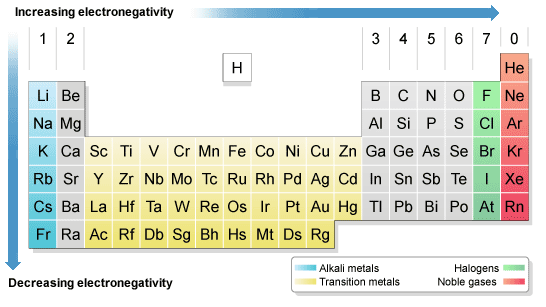
Periodic Table with Electronegativity
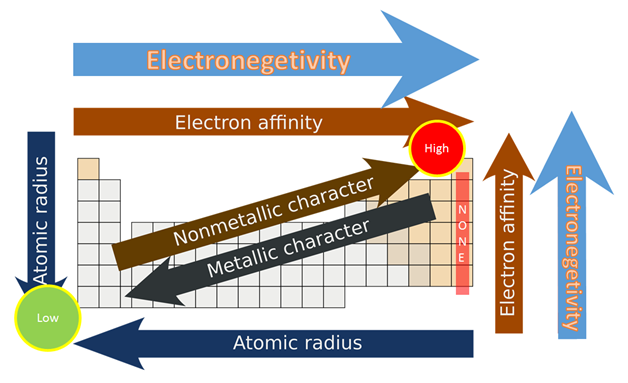
As we see in the periodic table electronegativity is already shown. When we move from left to right the atomic radius of the element decreases, but inside, ionization is an increases, and if we go in deeper then electronegativity increases. And if we move from top to bottom the atomic radius increases, the ionization energy decreases, and on another side electronegativity also decrease. You may also see that their noble gas have not any electronegativity. They also do not make any bonds with anyone, the reason behind this is outer shell electrons are full of 8 electrons, and they have very little tendency to participate in chemical reactions.
Electronegativity Chart of Periodic Table
If I talk more about periodic table electronegativity then it lights different elements. It varies a widely then looking at ionic bonds where a metal or non- metal combine. In case bonding between nonmetals, it would be considered as a covalent bond, when metal-metal interact then the results come out with metallic bonding.
Finally, I try to provide you chart, table, and whole content about electronegativity. Also complete information about it. If you feel it’s good then share it also with your friends, to provide the knowledge. If you have any suggestions then comment below.