Study the Rutherfordium valence electrons and develop a thorough understanding of this chemical element for your purpose. Here in the article, in periodic table we are going to cover up the useful information on the valency and the valence electrons of this particular element. We believe the article would come in handy for all the scholars and the other enthusiasts of Chemistry.
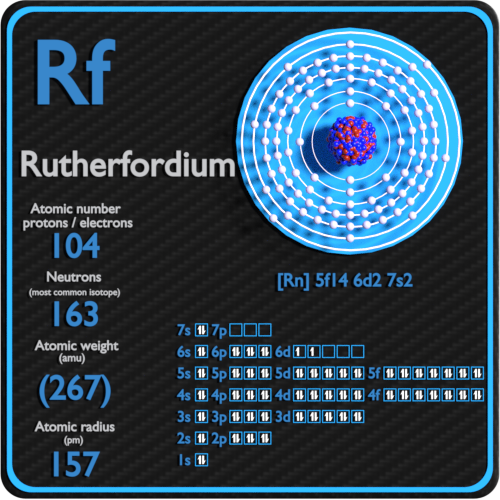
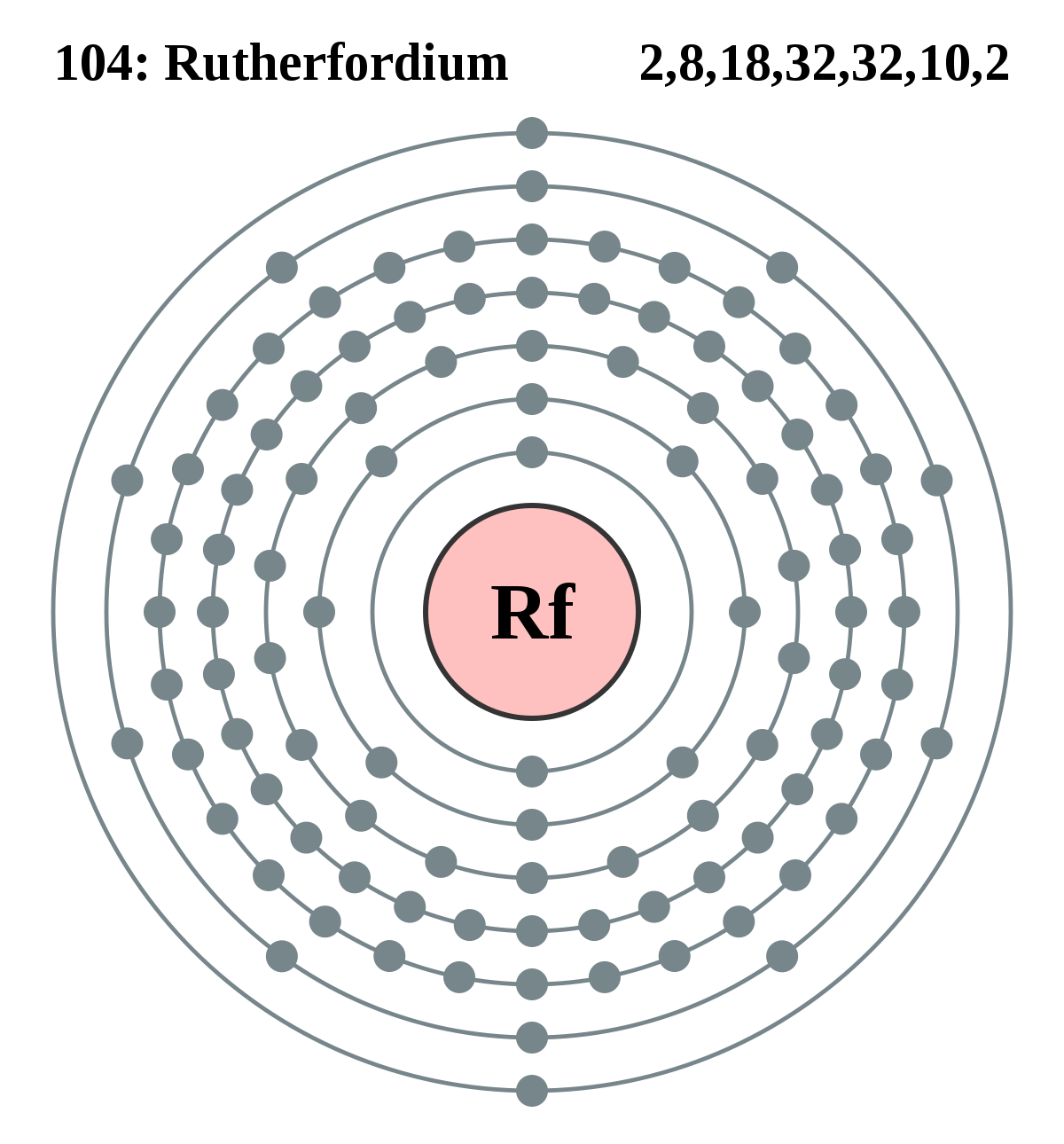
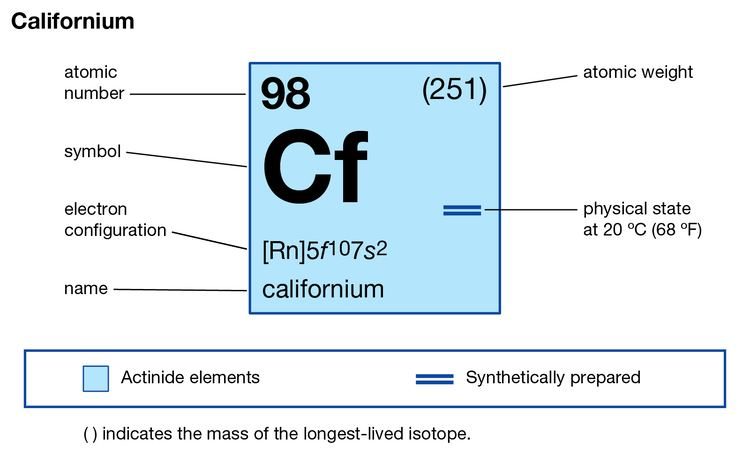
In Chemistry the rutherfordium is the chemical element that comes with its atomic number 104 and the symbol of Rf. The element is purely synthetic in its nature and was discovered by a famous scientist of New Zealand in the lab. Being the synthetic element there is no availability of rutherfordium in nature with its free form. It belongs to the category of the transition elements from the d group category. The element has very high decaying properties due to which the element is available only in a limited quantity. Besides, it’s a highly expensive element that is relevant only in the research phase as of now.
How Many Valence Electrons Does Rutherfordium Have?
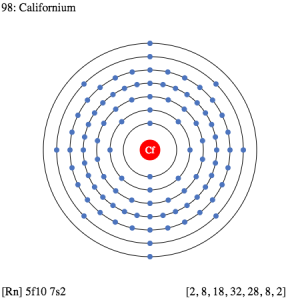
Well, the valence electrons of the rutherfordium can be written as 6d27s2 for simplification purposes. The valence electrons represent the actual numbers of the electrons that are available in the outer shell of the rutherfordium. These electrons constitute a significant part of the element and are also useful in determining the valency of this element. It further takes part in the combining process of the element with the other chemical elements.
Rutherfordium Valence Electrons Dot Diagram
The dot diagram is something that is highly useful for the broader representation of the chemical’s valence electrons. The dots in this diagram represents the actual number of valence electrons. The diagram is further useful in figuring out the other significant properties of the element for the experimental and the general view of the element.
Valency of Rutherfordium
Valency is the combining capacity of the element and every element has its own valency. It’s actually the process of the chemical reaction during which the atom of an element either gains or loses the electron to have a stable electron configuration. So, in the case of rutherfordium, we have the valency of 4 (four) and you can check the same in its periodic table