Dmitri Mendeleev has invented the periodic table which arranges all the chemical elements in a proper way that is in the order of increasing atomic number, electron configuration, and recurring chemical properties.
How Did Mendeleev Arrange the Periodic Table
Mendeleev got to know that the chemical and physical characteristics of elements somehow related to the mass of the atom in a ‘periodic’ way, so he arranged them in a manner that groups of elements with the same characteristics fell into vertical columns in his periodic table. Predictions and gaps in this way of arranging elements mean there gaps in his horizontal rows that called ‘periods’. But Mendeleev did not see this as a problem and thought that it simply means the elements in the gaps had not yet discovered.
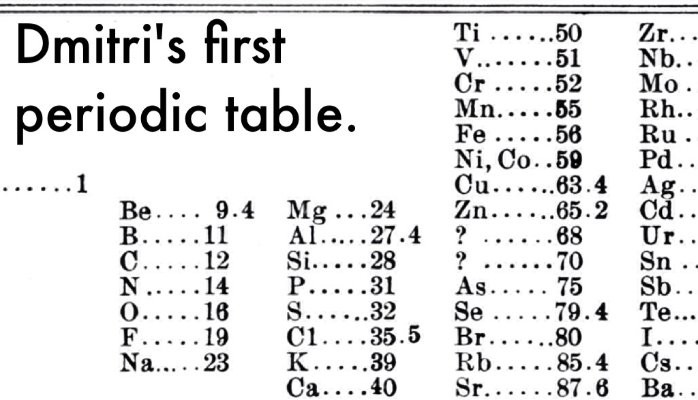
He also worked on the atomic mass of the missing elements, and so predict their characteristics. And when these elements discovered, Mendeleev proved to be right. Let’s take an example, he predicted the characteristic of an undiscovered element that should set and fit below aluminium in the periodic table. When this element discovered in 1875 its properties found to be same as Mendeleev’s predictions and that element gallium.
Dmitri Mendeleev Periodic Table PDF
You can find the PDF of Mendeleev periodic table below:
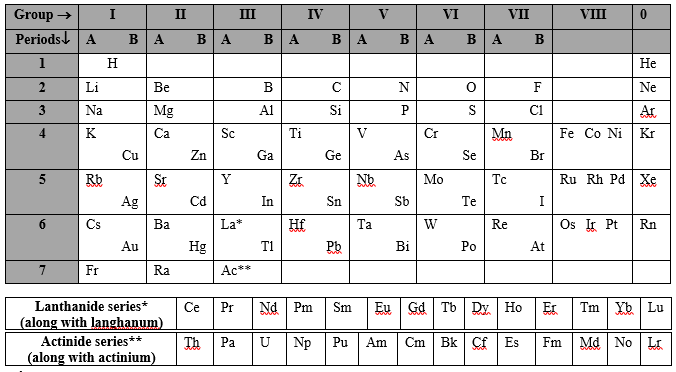
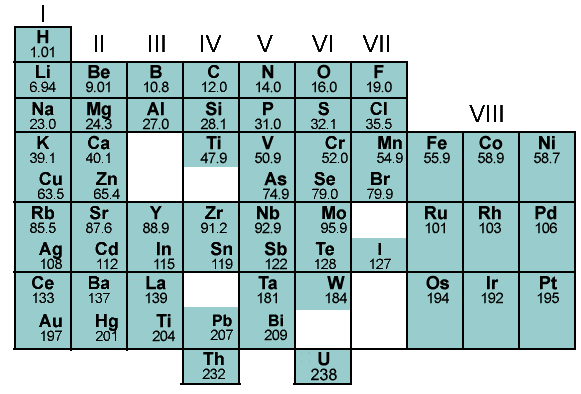
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og
How many Elements did Mendeleev Periodic Table have?
The original version of the periodic table created by Dmitri Mendeleev in 1869 consisted of 63 elements. Mendeleev organized the elements based on their atomic weights and properties, grouping them into columns (groups) and rows (periods) with similar characteristics. He left gaps in the table for elements that were yet to be discovered.
Since Mendeleev’s time, the periodic table has evolved and expanded as new elements have discovered. As of my knowledge cutoff in September 2021, the periodic table includes 118 confirmed elements, with the most recent additions being elements 113 (Nihonium), 115 (Moscovium), 117 (Tennessine), and 118 (Oganesson), which were officially recognized in 2016.
It’s important to note that the number of elements in the periodic table may change in the future as scientists continue to discover and synthesize new elements beyond atomic number 118. Check out other related posts:- Modern Periodic Table of Elements, What is the Electron Cloud Definition.
Achievements of Mendeleev Periodic Table
Dmitri Mendeleev’s creation of the periodic table considered one of the most significant achievements in the field of chemistry. Here some notable achievements associated with Mendeleev’s periodic table:
- Organization of elements: Mendeleev arranged the known elements based on their atomic weights and properties, creating a systematic framework that allowed for easy classification and identification. His periodic table grouped elements with similar properties together, making it easier to understand the relationships between elements and predict the properties of undiscovered elements.
- Prediction of new elements: Mendeleev’s periodic table had gaps in certain positions, suggesting the existence of yet-to-be-discovered elements. Based on the patterns and properties of neighboring elements, Mendeleev accurately predicted the properties of these missing elements. When the predicted elements were later discovered and their properties matched Mendeleev’s predictions, it confirmed the validity and usefulness of his periodic table.
- Correcting atomic weights: Mendeleev noticed inconsistencies in the atomic weights of some elements. He proposed revisions to the atomic weights of certain elements based on their chemical properties and positions in the periodic table. His adjustments, later confirmed by more precise measurements, helped refine our understanding of atomic weights and brought greater accuracy to the periodic table.
- Contribution to chemical nomenclature: Alongside the periodic table, Mendeleev made significant contributions to chemical nomenclature. He introduced the concept of valence, which described the combining capacity of elements, and he formulated rules for naming compounds. His work in chemical nomenclature helped establish a consistent and systematic approach to naming and understanding chemical compounds.
Mendeleev’s periodic table revolutionized the study of chemistry. Providing a framework that organized the elements and their properties in a logical and predictive manner. His achievements laid the foundation for further advancements in the field. And continue to be fundamental to our understanding of the elements and their relationships.
Mendeleev Periodic Table Based On
The Mendeleev periodic table is based on the principle that the properties of elements periodic functions of their atomic numbers. It organized by atomic weight, grouping elements with similar properties together. The key factors include:
- Atomic Weight: Elements arranged by increasing atomic weight, revealing patterns in their chemical properties.
- Grouping by Similarity: Elements with similar properties grouped together in columns (groups).
- Periodic Repetition: Properties of elements repeat periodically across rows (periods) in the table.
- Predictive Power: Mendeleev’s table successfully predicted the existence and properties of undiscovered elements.
Mendeleev’s periodic table revolutionized the understanding of elements and their properties, providing a systematic framework for organization and prediction.
Leave a Reply