Electron Configuration For Lawrencium: Electron Configuration has been the core process of researching a chemical element into atomic physics. This process of electron configuration helps the scientists, and the chemist to study the chemical element from every aspect so that it can be used in its appropriate way.
Today in this article we are going to discuss the electron configuration of Lawrencium chemical element.
Electron Configuration For Lawrencium
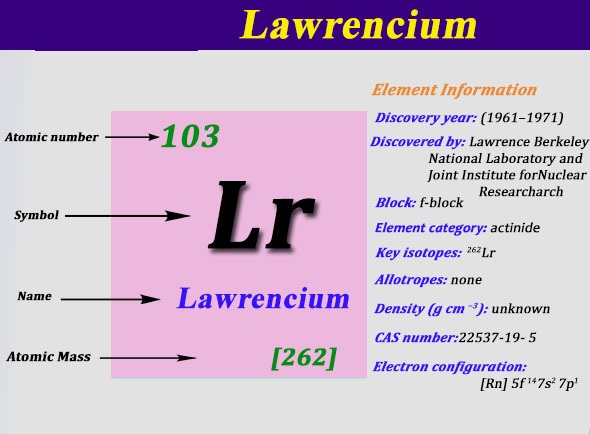
Lawrencium is the chemical element which belongs to the family of the Actinide series and is the last member of the series. This element is symbolized by the Lr.
When we say the term electron configuration for Lawrencium it simply implies that here we are going to make the distribution of the electrons of the Lawrencium chemical element, in the context of molecular or the atomic orbital…
Lawrencium chemical element was basically synthesized by the Albert GHIORSO and being the Synthesized element it is not found naturally in the environment rather created by the nuclear bombarding. This element is highly radioactive and for this reason, this element is possible to be used only in the scientific research work only.
What is the Electron Configuration of Lawrencium?
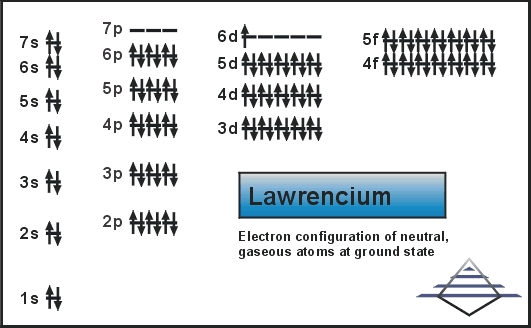
The electron configuration of the Lawrencium is written as [Rn] 5f14 6d1 7s2
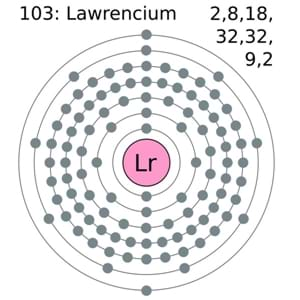
The Lawrencium holds the 2,8,18,32,32,9,2 electron shells. Further, it has the 103 electrons and the same 103 Protons.
The Density of the Lawrencium is 20 degrees Celsius and it has the solid form. This element has the 10 isotopes and their only half of the life is yet known.
How Many Valence Electrons Does Lawrencium Have
Lawrencium is the chemical element which has the 103 electrons and also the 103 Protons. Out of these 103 electrons, only three are available as the valence electrons hence it can be said that the Lawrencium has 3 Valence Electrons.
The atomic number of the Lawrencium is 103 with the atomic weight of the 262. This chemical element can be melted at the temperature of 1627 degree Celsius. It has the +3 oxidation states and the Lawrencium is considered to be the member of the Actinide series.
Lawrencium Number of Valence Electrons
As we have already mentioned above that this Lawrencium chemical has the 103 of the electrons, and out of which the 3 are the valence electrons for this chemical element. This chemical element is located in period 7 and the block f of the periodic table.
If we talk about the atomic structure of this chemical element then it has 103 electrons, 103 protons, and the 159 of the Neutrons. The atomic radius of the Lawrencium is 2.46 A and the covalent radius is 1.61 A.
This chemical element is the member of the Actinide series and it is the heaviest element of this series.
Leave a Reply