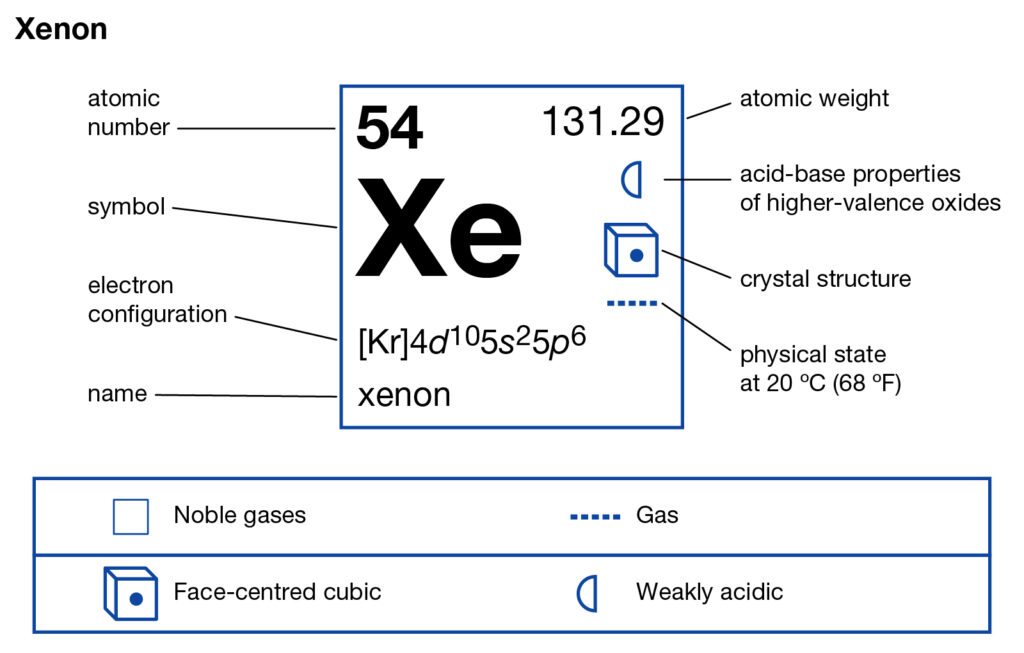
Get to understand the Xenon Valence Electrons and more about the element. In the article below we will explore the element with in-depth insight. Xenon is a typical chemical element in the language of chemistry. The chemical has the atomic number as 54 and the symbol as Xe. Xenon belongs to the family of noble gas and has significant availability in the atmosphere. It has the physical appearance of the dense, colorless and odorless element.
How many valence electrons does Xenon have?
In general characteristics Xenon is an unreactive chemical element, however, it may react on some occasions. It has another form of occurrence such as from mineral springs. So, Xenon has its availability in the earth’s atmosphere. It’s extracted in the air separation process from the oxygen and nitrogen.
Xenon although has significant availability in the atmosphere yet it’s quite expensive. So, Xenon generally has a higher cost than other noble gases. Xenon has a number of usages in the commercial and medical domain. Most of the light-emitting devices, lamps, lasers contain Xenon gas within them.
Further usage of Xenon includes the production of Anesthesia in the medical domain. The anesthesia made of Xenon is generally expensive than the conventional ones. Sports doping etc are the other misuses of Xenon by the athletes. It increases the performance of athletes on temporary basis for the time being.
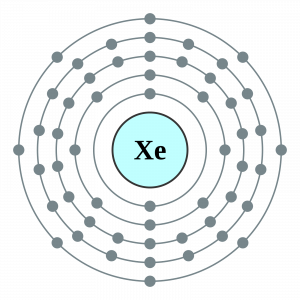
Xenon Valence Electrons Dot Diagram
You can here understand the Xe valence electrons representation. The dot diagram represents the numbers of Xenon valence electrons.
So, It draws the numbers of valence electrons around the symbol of Xenon. You can further understand the chemical interaction of valence electrons by the diagram.
Valency of Xenon
Well, Xenon has the variable valency of +2,+4,+6 in a different scenarios. It’s the combining capacity of Xenon to combine with other chemical elements.
Leave a Reply