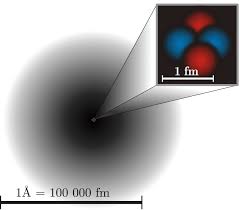
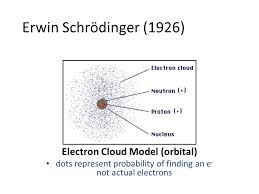
How To Find The Electron Cloud Model Definition Chemistry? If you are a chemistry student then knowing the electron cloud model is very important for you. It was given by Erwin Schrödinger and Werner Heisenberg in 1926. According to this model, the electrons do not revolve around their orbitals in a specific path.
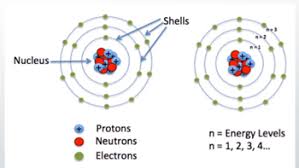
Rather they are accumulated at specific spots near the nucleus like a cloud. This theory is quite different from the earlier Bohr’s atomic model given by Neil Bohr, according to which, electrons were assigned to different shells in an atom.
How To Find The Electron Cloud Model Definition Chemistry?
The electron cloud model, also known as the quantum model or wave-mechanical model, is a fundamental concept in chemistry that describes the behavior and location of electrons in an atom. To understand this model’s definition in chemistry, it is essential to delve into the realm of quantum mechanics, where electrons are treated as both particles and waves.
At its core, the electron cloud model represents the probability of finding an electron in a particular region around the nucleus. Unlike the earlier Bohr model, which depicted electrons as discrete orbits, the electron cloud model presents electrons as existing in regions known as orbitals. These orbitals are three-dimensional spaces around the nucleus where the probability of finding an electron is highest. The electron cloud model provides a more accurate and sophisticated understanding of atomic structure and bonding.
To comprehend the electron cloud model, one can explore the Schrödinger equation and its solutions, which are mathematical functions that describe the behavior of electrons as waves. These solutions yield different energy levels and shapes of orbitals, corresponding to different quantum numbers. By studying these quantum numbers and orbitals, chemists can predict an electron’s energy, position, and likelihood of being found in a specific part of an atom.
In conclusion, the electron cloud model in chemistry describes the probabilistic behavior of electrons around an atom’s nucleus. Understanding this model involves delving into quantum mechanics and the Schrödinger equation to comprehend the concept of orbitals and their associated quantum numbers. Embracing the electron cloud model enables chemists to gain a more accurate and comprehensive view of atomic structure and chemical properties.
What is the Difference Between Electron Cloud and Orbital?
The terms “electron cloud” and “orbital” often used interchangeably, but they refer to distinct concepts in the realm of atomic structure and quantum mechanics. Understanding the difference between these two concepts is essential to grasp the intricacies of electron behavior within an atom.
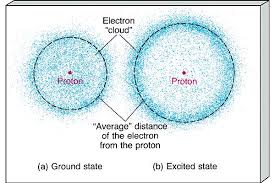
The electron cloud refers to the region around an atom’s nucleus where electrons most likely to found. It a three-dimensional space where the probability density of finding an electron is highest. The electron cloud is a fundamental concept in the electron cloud model, also known as the quantum model, which describes electrons as both particles and waves. This model emphasizes the probabilistic nature of electrons’ positions rather than their fixed orbits.
On the other hand, an orbital is a specific mathematical function that describes the behavior of an electron in an atom. Each electron in an atom described by a unique orbital that corresponds to a particular set of quantum numbers. These quantum numbers define the electron’s energy, angular momentum, and orientation within the atom. Orbitals come in different shapes, such as s, p, d, and f orbitals, each with distinct characteristics.
What Does the Electron Cloud Represent?
The electron cloud is a crucial concept in atomic physics and chemistry. It represents the three-dimensional probability distribution of an electron’s position around an atom’s nucleus. In the electron cloud model, electrons treated as both particles and waves, and their behavior described by mathematical functions called wave functions or orbitals.
The concept of the electron cloud arose as a departure from the classical Bohr model, which depicted electrons orbiting the nucleus in fixed paths. Instead, the electron cloud model acknowledges the inherent uncertainty in predicting an electron’s exact position at any given time. Instead of rigid orbits, the model suggests that electrons exist in regions of space with varying probabilities of finding them.
The shape of the electron cloud determined by the energy level and quantum numbers associated with the electron. Each electron in an atom has a unique set of quantum numbers, which dictate its energy, angular momentum, and orientation. These quantum numbers define the size, shape, and orientation of the orbitals within the electron cloud.
What is Electron Cloud in Chemistry?
In chemistry, the electron cloud refers to the region surrounding an atom’s nucleus where electrons most likely to found. The concept of the electron cloud emerged from the development of quantum mechanics and the electron cloud model, which revolutionized our understanding of atomic structure.
According to the electron cloud model, electrons do not follow discrete orbits like planets around the sun, as suggested by the Bohr model. Instead, they exist in three-dimensional spaces called orbitals, where the probability of finding an electron is highest. Each electron in an atom described by a unique set of quantum numbers, determining the energy level and shape of its orbital.
The electron cloud plays a vital role in chemical bonding. During chemical reactions, atoms interact by either sharing or transferring electrons. The probability of finding electrons in specific orbitals affects the atoms’ reactivity and the nature of the chemical bonds they form. For example, atoms with unfilled outermost orbitals tend to react more readily, as they seek to achieve a stable electron configuration.