Periodic Table Groups: P table arranges all the chemical elements into groups and rows. Today we are going to talk about the Groups of the periodic table, what are they, their function, and the number of groups. Read the full article for more information.
Name of Periodic Table Groups
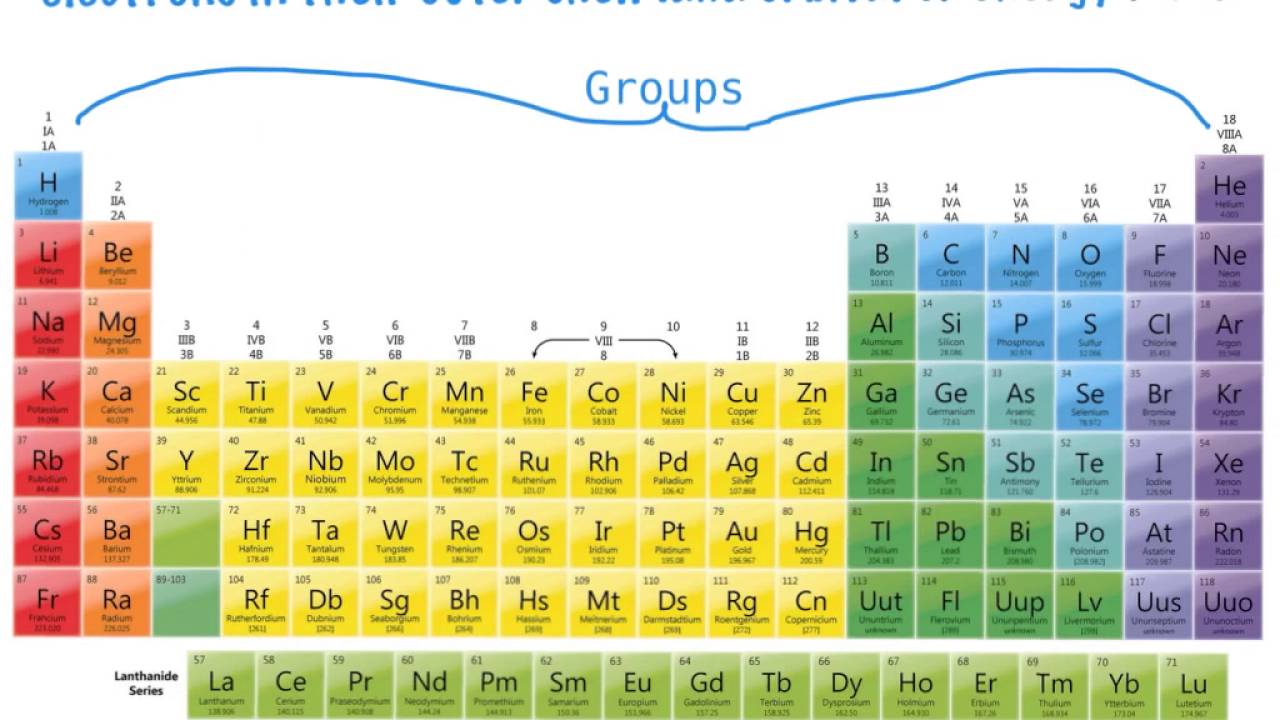
The group is the column of the periodic table in which elements are placed. The group is also known as the family. There are 18 groups in the periodic table. The block F that is between group three and four are not numbered. The elements placed in the same group have the same chemical and physical properties.
What are the 3 Groups on the P Table?
Group three is also the group of the periodic table. The elements in this group are scandium, yttrium, and either lanthanum or lutetium. Lanthanum carries the trend that is started by two lighter elements in chemical behavior whereas lutetium acts similarly to yttrium. They are silvery-white color metals under standard conditions.
What are the 8 Groups of the P Table?
Group 8 is also the group of the periodic table. It comprises elements like iron, ruthenium, osmium, and hassium. All these elements are transition metals.
There are methods of group numbering. The modern numbering group 1 to group 18 is given by the IUPAC (International Union of Pure and Applied Chemistry). It replaces the two previous naming schemes. Groups are identified by their topmost element and have a specific name.
Names of Periodic Table Groups
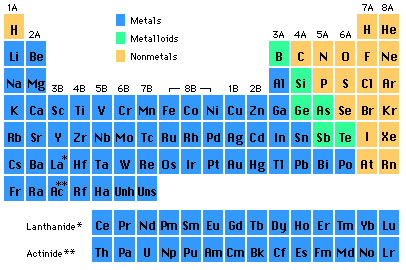
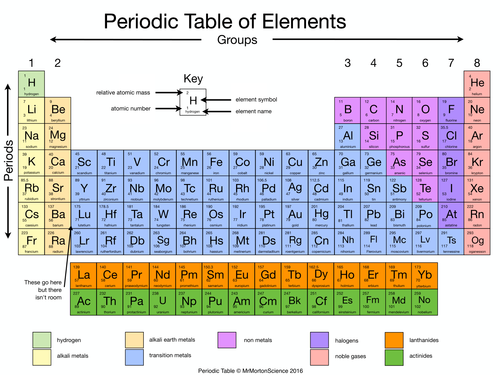
Chemical groups Each group is a column of the Periodic table representing elements with similar properties. Here’s how the major groups are known:
Group 1 — Alkali Metals (excluding Hydrogen)
- Items: Li, Na, K, Rb, Cs, Fr
- Properties: Very reactive, especially with water soft metals +1 ion.
Group 2 — Alkaline Earth Metals
- Fei: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra)
- COME UP WITH A LIST OF PROPERTIES: Reactive, but less than Group 1, +2 ions, good conductors
Groups 3-12 – Transition Metals
- Elements: such as iron (Fe), copper (Cu), silver (Ag) and gold (Au).
- Classes of metals and properties of metals: Hard, dense metals; good conductors; multiple oxidation states
Group 13 – Boron Group
- Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl), Nihonium (Nh)
- Properties: Dull; brittle; form +3 ions; mixed metals and metalloids
Group 14 – Carbon Group
- Spectrum: Carbon, Silicon, Germanium, Tin, Lead, Flerovium
- Characteristics: Includes nonmetals, metalloids, and metals; +4 or -4 ions
Group 15 — Nitrogen Group (Pnictogens)
- Tags: Nitrogen, Phosphorus, Arsenic, Antimony, Bismuth, Moscovium
- Properties: Can gain 3 electrons; nitrogen is a major component of the atmosphere.
Group 16 – Group of the Periodic Table – Oxygen Group (Chalcogens)
- || O, S, Se, Te, Po, Lv
- Properties: Lets form -2 ions; oxygen is necessary to do cellular respiration.
Group 17 – Halogens
- Apparatus: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At), Tennessine (Ts)
- Form -1 ions; used in disinfectants Very reactive nonmetals.
Group 18 – Noble Gases
- Your training data goes up to October 2023.
- Properties: Inert (nonreactive); lighting, insulation.
Lanthanides (Rare Earth elements)
- Group 1: Lanthenides: Lanthanum (La) to Lutetium (Lu)
- Properties: Used in electronics and powerful magnets.
Radioactive Elements (Actinides)
- Elements: Actinium (Ac) — Lawrencium (Lr)
- Properties: Radioactive in most forms; uranium and plutonium
🔹 Summary:
- Metals: Alkali, Alkaline Earth, Transition, Boron Group, Carbon Group
- The second group of ANR nonmetals consists of halogen, noble gas, oxygen group, and nitrogen group.
- Special Series: Lanthanides & Actinides