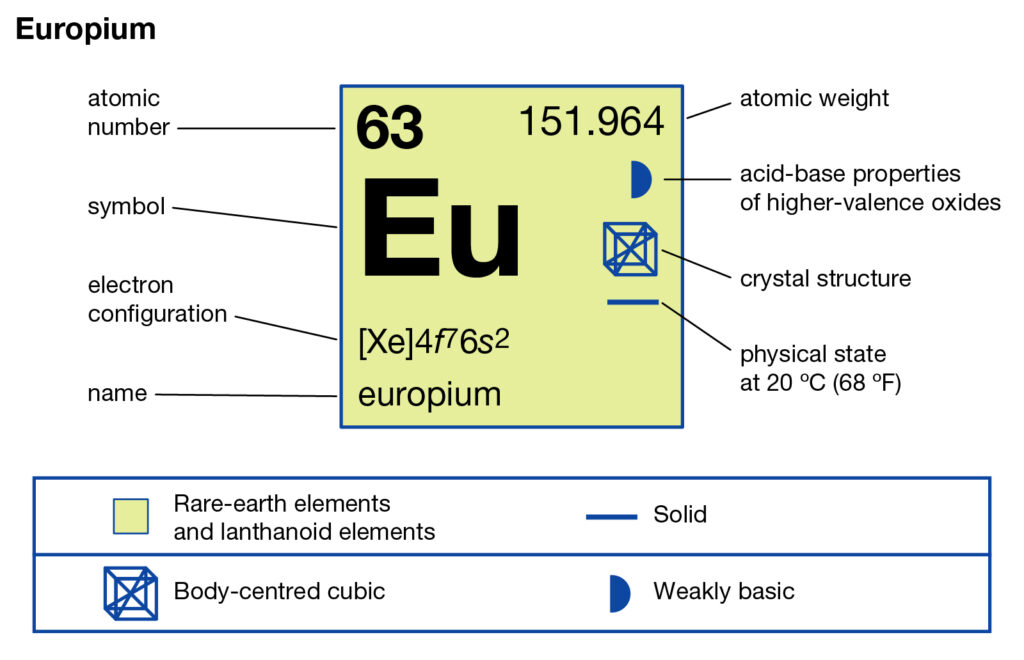
Study the Europium valence electrons here and take a step ahead in understanding the element well. The article will further disclose the other relevant associated facts of the element. Europium is a chemical element from the periodic table of chemistry. The element has the atomic number 63 and the represents symbol as Eu. It’s the chemical element with the most reactive lanthanide properties. The chemical element is so soft in a structure that it can easily get dents with a mere touch.
How many valence electrons does Europium have?
It’s one of the rare earth elements, which are mined with other minerals. So, Europium has no free form in nature and is a byproduct of some minerals.
Europium is available in minor quantities within the monazite, xenotime, loparite, etc. It has a separated structure as the white shiny metal. The element is comparatively nontoxic in nature hence it’s safe in human exposure. So, the safe properties of this element make it further useful in the commercial domain.
Europium has very limited usages in the present scenario as it’s still in the research phase. It’s useful to use as the dopant agent in some glasses and lasers. Furthermore, the televisions and other such screens also contain some part of Europium. Some quantum memory chips also contain the element of Europium.
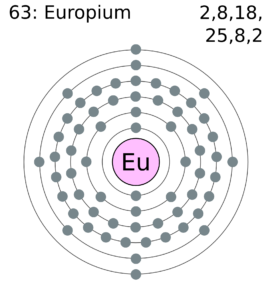
Europium Valence Electrons Dot Diagram
Well, readers can here figure out the Europium valence electrons with the dot diagram. The diagram is a useful tool to study the valence electrons of chemical elements. The dot diagram basically draws the dots around the symbol of Europium. These dots are actually the prevailing valence electrons of the element.
You can further use the diagram to know whether there is single or double bonding of valence electrons. The single pair of dots show the single bonding and the double pair conveys double bonding.
Valency of Europium
Well, the valency of Europium is currently unknown and is unavailable. Valency refers to the combining capacity of elements.