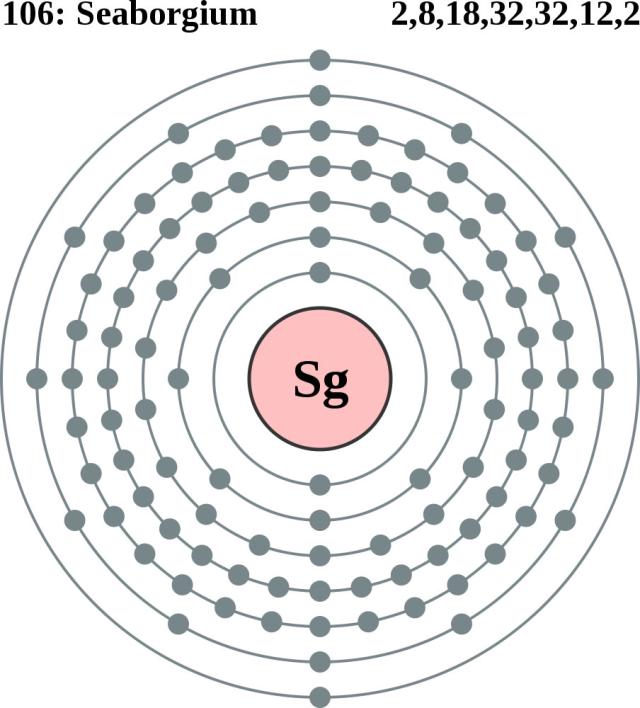
Do you want to study the Seaborgium valence electrons? Well, you can start it from here as we are going to make our discussion on the same in the article ahead. The article periodic table contains significant information on the valency and the valence electrons of this particular element. Seaborgium is the chemical element that has the atomic number of 106 and the symbol of Sg.
The element was discovered by the American scientist Glenn T Seaborg in 1974 within the laboratory. The element is therefore the synthetic in nature and has no free occurring form in nature. Being a radioactive element it has the harmful radioactive element and is available only for laboratory usage. Just like other synthetic elements Seaborgium is also quite expensive in its nature and therefore it’s suitable only for laboratory usage.
How Many Valence Electrons Does Seaborgium Have?
Seaborgium has the six valence electrons in the outer shell of the atom that take part in the combining process of elements. The valence electrons number is crucial in nature since it depicts the other properties of the element. For instance, the valency of the element is also associated with its valence electrons. We have the specific number of the valence electron for any element at a time. One can also check the valence electrons of Seaborgium in the periodic table of the element.
Seaborgium Valence Electrons Dot Diagram
Lewis dot diagram is something that we highly suggest to every scholar of chemistry who is exploring the chemical elements. The diagram is significant since it shows up the actual numbers of the valence electrons for the atom in the form of dots. These dots are highly effective when it comes to the top-notch visualization of these valence electrons. So, feel free to take a broader visualization of the Seaborgium valence electrons with the dot diagram.
Valency of Seaborgium
The combining capacity of the Seaborgium with the other element is what we call its valency. In the case of the Seaborgium, we have the valency of 5 & 4 in their respective manner. It simply implies that this element may gain or lose these numbers of electrons to gain its stable electron configuration.