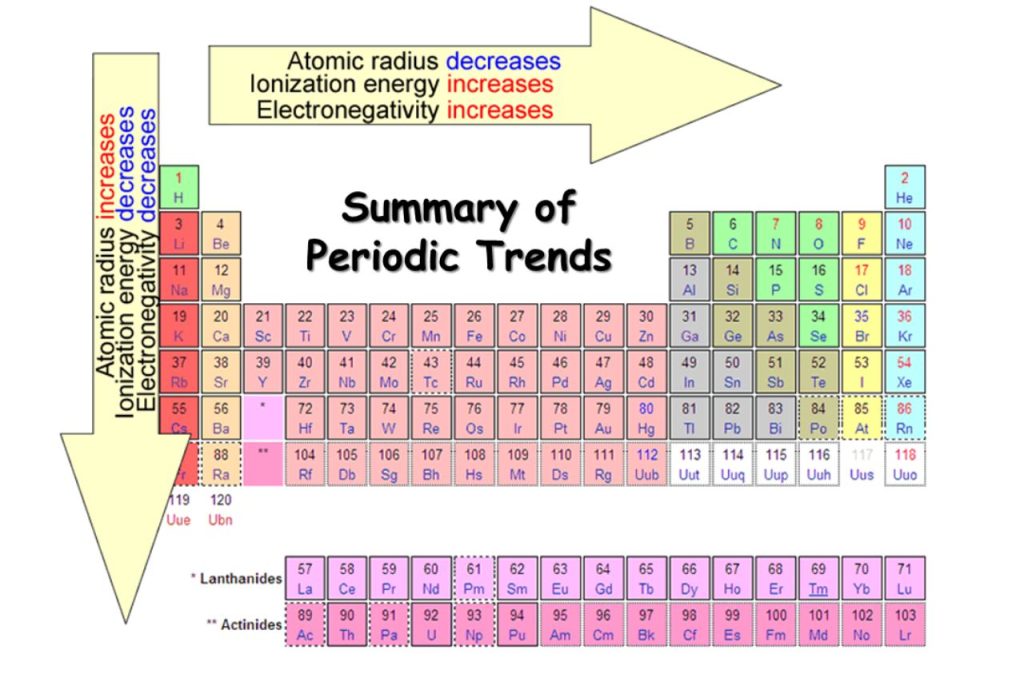
Mysteries of the Periodic Table – The Periodic Table is a comprehensive chart that organizes all of the known chemical elements in Chemistry. It is one of the most important tools in chemistry, as it allows scientists to predict the properties of elements and their behavior in chemical reactions.
Mysteries of the Periodic Table
However, there are still some mysteries that surround the Periodic Table which can be understood with the help of Chemistry tuition.
Island of Stability
One of the most intriguing mysteries of the Periodic Table is the existence of an “island of stability” beyond the known elements. This island is predicted to exist between elements with atomic numbers 120 and 130. These elements are thought to be more stable than the heavier elements that have already been synthesized.
Superheavy Elements
Scientists have been able to synthesize elements up to atomic number 118. However, these elements are very unstable and only exist for a fraction of a second. It is not yet known if it will be possible to synthesize elements that are even heavier.
Electron Configuration
The electron configuration of an element is the arrangement of its electrons in orbitals. The electron configuration is determined by the number of protons in the nucleus of the atom. However, there are some elements that have electron configurations that do not fit the expected pattern.
Group 8
Group 8 of the Periodic Table is known as the noble gases. These elements are all very stable and do not react with other elements. However, there are some elements in Group 8 that have been predicted to be unstable.
The Future of the Periodic Table
The Periodic Table is constantly being updated as new elements are discovered. It is possible that the Periodic Table will continue to grow and change as we learn more about the universe.
These are just a few of the mysteries that surround the Periodic Table. As scientists continue to learn more about chemistry, they may be able to solve these mysteries and unlock even more secrets about the universe.