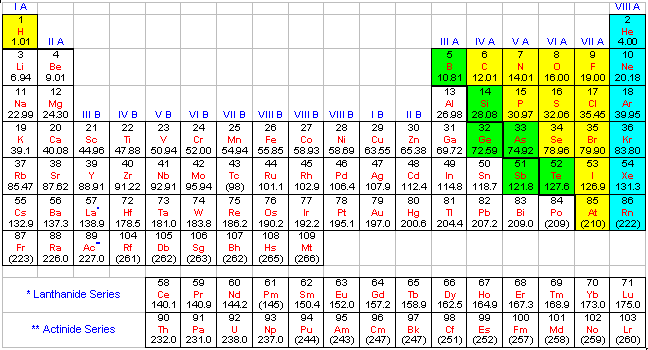
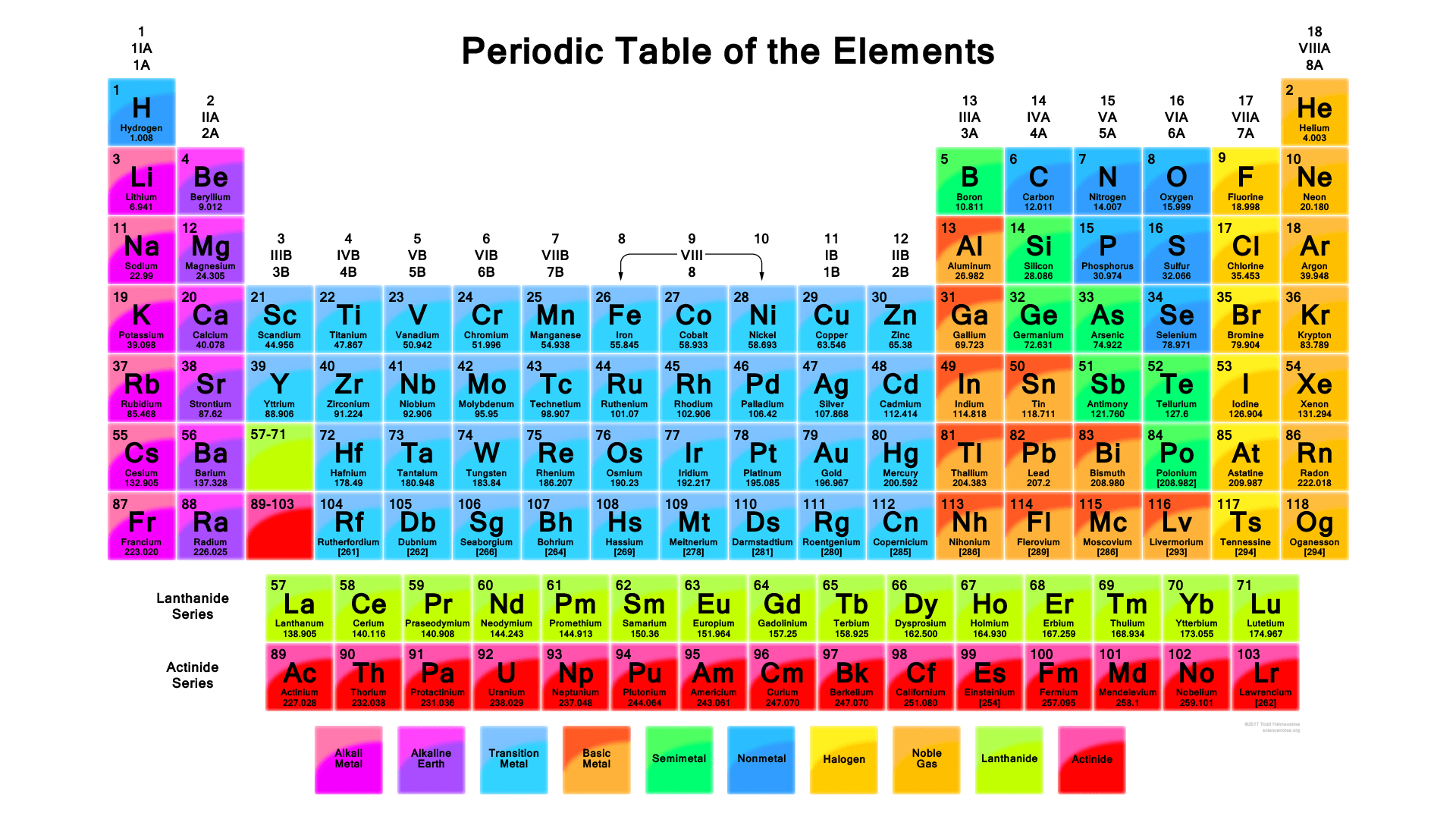
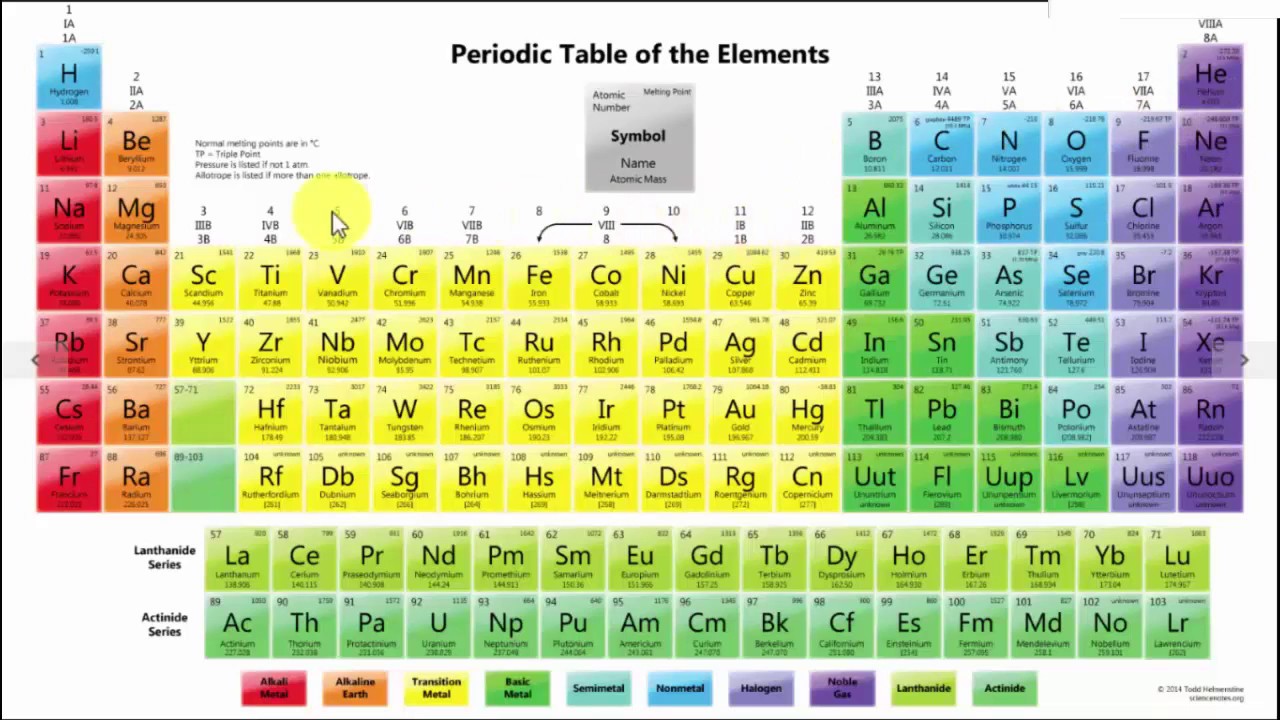
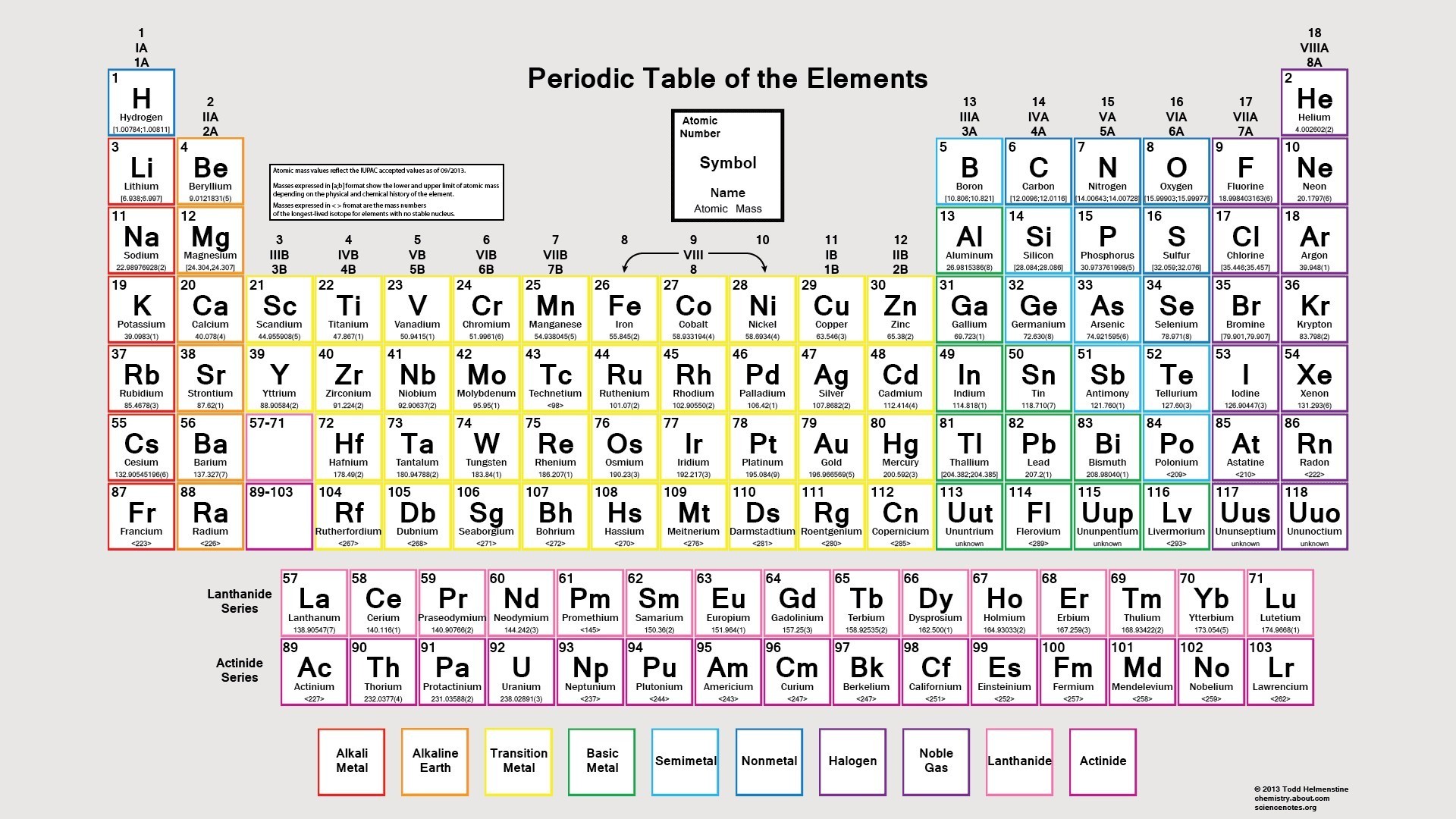
The modern periodic table is a tabular representation of all the chemical elements in the order of their atomic number, their electron configuration and their recurring chemical properties. This is the Periodic Table Definition.
What is the Modern Periodic Table
Who Has Given The Modern Periodic Table?
Dmitri Mendeleev, a Russian chemist has given the first periodic table. He arranged all the chemical elements in order of their atomic mass corresponding to their relative molar mass. He arranged the elements in the increasing order of their atomic number.
How Are The Elements Arranged In The Modern p Table
The periodic table of elements put the entire chemical element in order. Elements are arranged from top to bottom and from left to right in increasing order of their increasing atomic number and coincide with increasing atomic mass. The rows in the table are called periods.
Element’s periodic number signifies the highest energy level an electron occupies. Electron’s number in a period increases as one moves down the periodic table. Therefore the energy level of an atom increases, the number of sub-level energy level also increases.
Modern P Table PDF
If you want to know about What is the modern p table of elements? And are looking for a periodic table of elements, you are in the right place because we are providing PDF. You can click and download it from below.
Modern Periodic Table Chart
If you are a chemistry student, you must have known about the modern table. The chart of the modern table that we are providing will surely help you to understand the arrangement in a better way. You can download the chart by clicking the option below.
Modern Periodic Table With Names
We are providing the modern p table along that contains both scientific name and common name along with their symbols. You can download this amazing table from below.
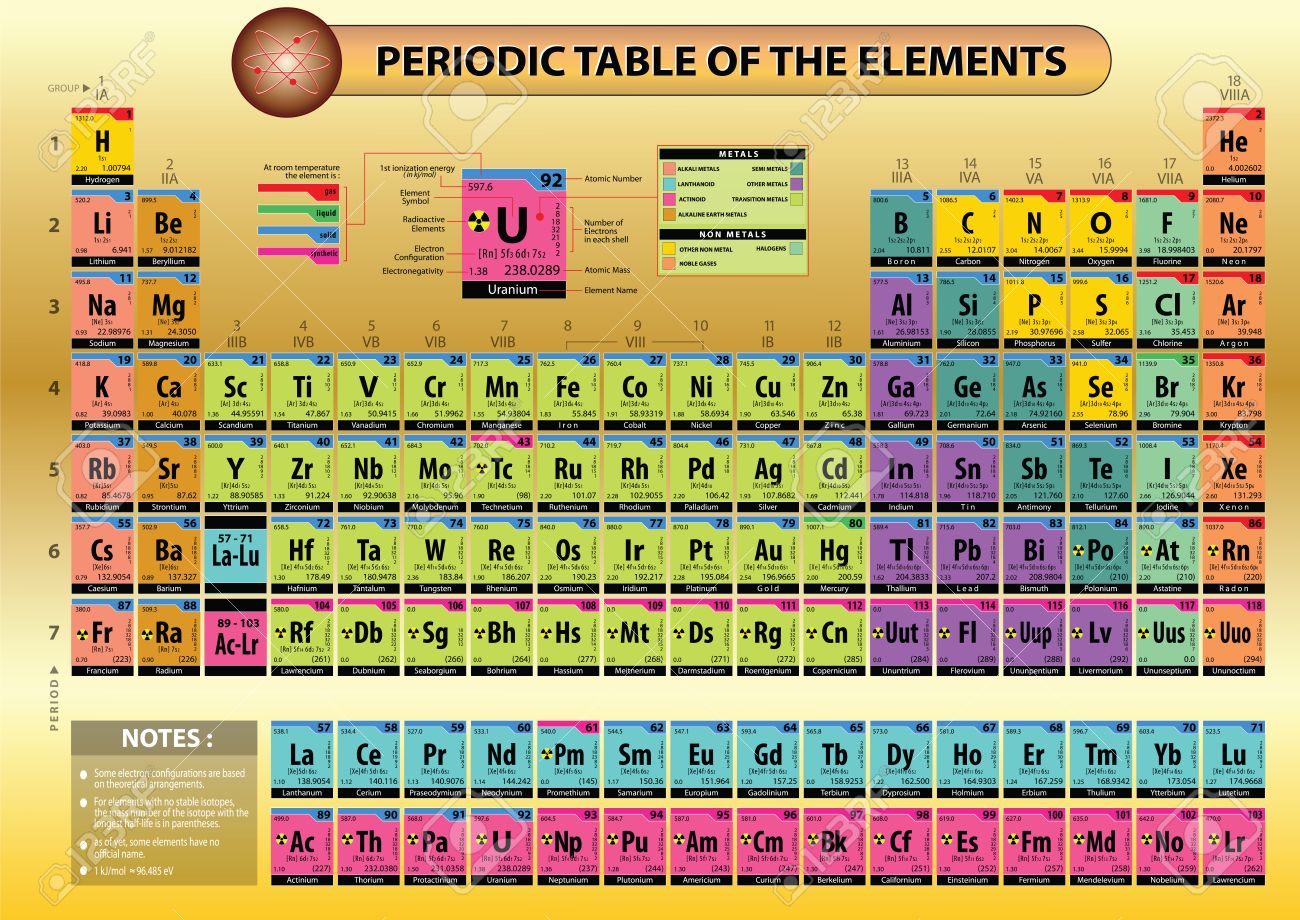
Modern Periodic Table With Atomic Mass
The periodic table that is provided below is not only arranged according to their atomic mass but it also contains the atomic mass of the elements.