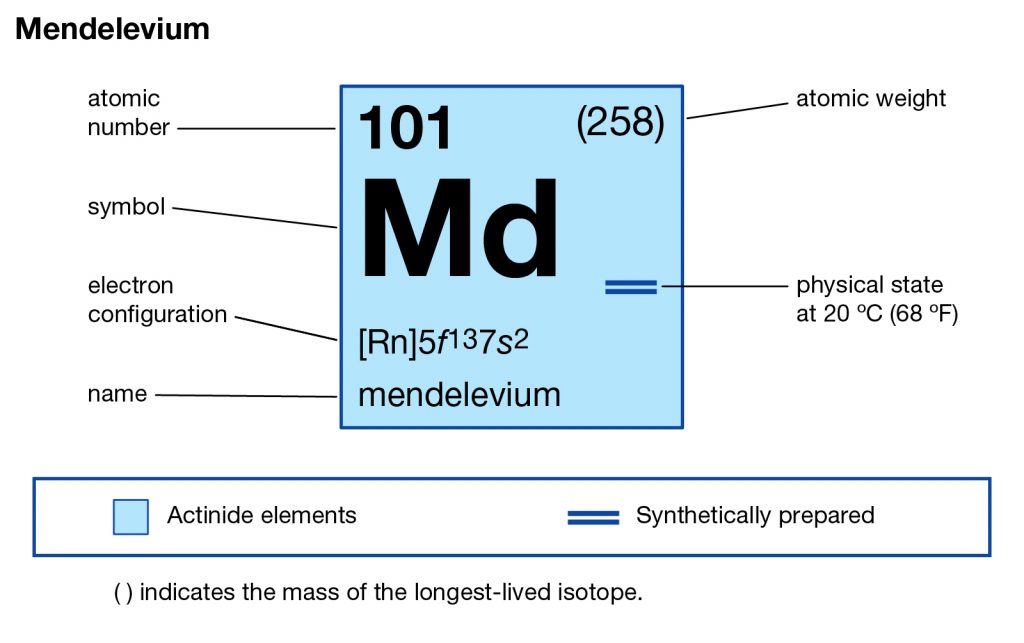
Get to study the Mendelevium valence electrons here and explore the significant properties of the element. Our scholar readers can here find the simple guide and information to study this chemical element for their general knowledge or the part of academics. In Chemistry Mendelevium is the chemical element that has the symbol of Md and the atomic number of 101. The element further belongs to the series of Actinide elements in the periodic table.
Mendelevium is synthetic in nature and can only be produced by the particle acceleration process. The element was first discovered in 1955 and the same approach is still useful to produce this element. Moreover, the element is still the subject of its research phase since it has no other practical usage as of now. Lastly, the element is quite expensive from the produced point of view and is also not safe for human exposure.
How Many Valence Electrons Does Mendelevium Have?
This particular element has the 3 (Three) valence electrons as an integral part of its outer shell. These electrons are known as the valence electrons due to their presence in the outer molecule. Valence electrons are significant since they form part of the combining process of the element. In a likewise manner they also determine the further significant properties of the element which become the subject of research later on.
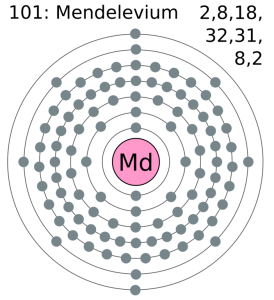
Mendelevium Valence Electrons Dot Diagram
If you are still feeling doubtful in your head regarding the valence electrons then you can refer to its dot diagram. This diagram is quite useful in the visualization of the valence electrons for the atom. As it shows the numbers of the valence electrons in the form of dots which become quite easy for the understanding purpose. So, we highly recommend our scholars go through the dot diagram for having a broad understanding of Mendelevium valence electrons.
Valency of Mendelevium
Mendelevium has the valency of 1 as its combining capacity to combine with the other elements. We call it the combining capacity since the Mendelvium can either lose or gain 1 electron to attain its stable state from the perspective of electron configuration. The valency shows the overall character of the Mendelevium in the periodic table of elements.