Neutron Symbol Mass and Charge: The neutron is an essential subatomic particle found in the nucleus of an atom. It plays a crucial role in determining an atom’s stability and nuclear reactions. This paragraph will explore the neutron’s symbol, mass, and charge, shedding light on its significance in the field of physics and atomic structure.
Neutron Symbol Mass and Charge
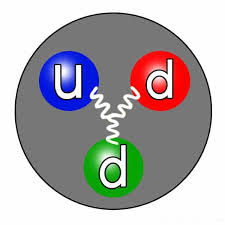
The neutron is one of the three fundamental particles that make up an atom, along with protons and electrons. It is electrically neutral, meaning it has no charge. The symbol used to represent a neutron is “n.” The mass of a neutron is approximately equal to that of a proton, with a mass of about 1.675 × 10^-27 kilograms, or 1.008 atomic mass units (u).
Neutrons play a crucial role in the stability of atomic nuclei. They found in the nucleus of an atom, tightly bound with protons. The number of neutrons in an atom can vary, giving rise to different isotopes of an element. Isotopes atoms of the same element that have different numbers of neutrons, thus varying in mass. The presence of neutrons in the nucleus helps balance the repulsive forces between positively charged protons, allowing the nucleus to remain stable.
Neutrons also important in various scientific fields, including nuclear physics and medicine. They commonly used in nuclear reactors, where they participate in nuclear reactions and contribute to the production of energy. In medicine, neutrons utilized in certain cancer treatments, such as neutron therapy, which involves targeting cancer cells with high-energy neutrons to destroy them.
In summary, the neutron symbol is represented by “n,” and it has no charge. Neutrons have a mass similar to that of protons and play essential roles in maintaining atomic stability and facilitating various applications in nuclear science and medicine. Learn more about Neutrons here:- Is a Neutron Positive or Negative Charge?.
What is the Correct Symbol for a Neutron
The correct symbol used to represent a neutron is “n.” This symbol was established in the early days of particle physics and is universally recognized. The symbol “n” effectively distinguishes neutrons from protons (symbol “p”) and electrons (symbol “e”) in atomic notation.
The neutron symbol “n” is used to denote the electrically neutral subatomic particle found in the nucleus of an atom. Unlike protons and electrons, which carry positive and negative charges, respectively, neutrons have no charge. This lack of charge allows neutrons to interact with other particles through the strong nuclear force, which is responsible for holding the nucleus together.
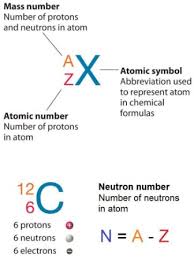
It’s important to note that the symbol “n” is not to confused with the letter “N,” which is sometimes used to represent the number of neutrons in an atom. The number of neutrons in an atom can vary, giving rise to different isotopes of an element. The symbol “n” refers specifically to the neutron particle itself, while “N” represents the neutron count within a specific atomic nucleus.
In conclusion, the correct symbol for a neutron is “n.” This symbol distinguishes it from other subatomic particles and represents its electrically neutral nature.
How to Find the Number of Electrons
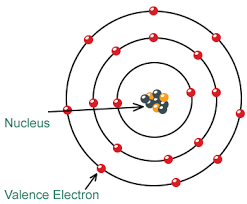
Determining the number of electrons in an atom is essential for understanding its atomic structure and chemical properties. The number of electrons in a neutral atom is equal to the atomic number of the element. The atomic number represents the number of protons in an atom’s nucleus and is denoted by the symbol “Z” on the periodic table.
To find the number of electrons in an atom, simply locate the element on the periodic table and identify its atomic number. For example, carbon has an atomic number of 6, indicating that it has six protons and, therefore, six electrons in its neutral state. Oxygen, with an atomic number of 8, contains eight protons and eight electrons. This relationship holds true for all elements in their neutral forms.
In certain cases, atoms may gain or lose electrons, forming ions. Positively charged ions, known as cations, have fewer electrons than protons, while negatively charged ions, called anions, have more electrons than protons. The charge of an ion can determined by the gain or loss of electrons relative to the atomic number.
In summary, the number of electrons in a neutral atom is equal to the atomic number of the element. This value can found on the periodic table and provides essential information about an atom’s electronic configuration and its involvement in chemical reactions.
Mass of Protons Neutrons and Electrons
Protons, neutrons, and electrons the three fundamental particles that make up an atom. Each particle has a different mass, and together, they contribute to the overall mass of an atom.
The mass of a proton is approximately 1.673 × 10^-27 kilograms, or 1.007 atomic mass units (u). Protons positively charged particles located in the nucleus of an atom. They have a mass that is approximately 1,836 times greater than that of an electron.
The mass of a neutron is very similar to that of a proton. It is approximately 1.675 × 10^-27 kilograms, or 1.008 atomic mass units (u). Neutrons electrically neutral particles also found in the nucleus of an atom. The similarity in mass between protons and neutrons is why they often considered to have roughly the same weight.
In contrast, electrons significantly smaller and lighter than protons and neutrons. The mass of an electron is approximately 9.109 × 10^-31 kilograms, or about 1/1836th the mass of a proton. Electrons negatively charged particles that orbit the nucleus of an atom in specific energy levels or electron shells.
The relative masses of protons, neutrons, and electrons play a crucial role in determining the overall mass of an atom. The mass of an atom is primarily determined by the combined masses of its protons and neutrons, while the electrons contribute relatively little to the total mass.