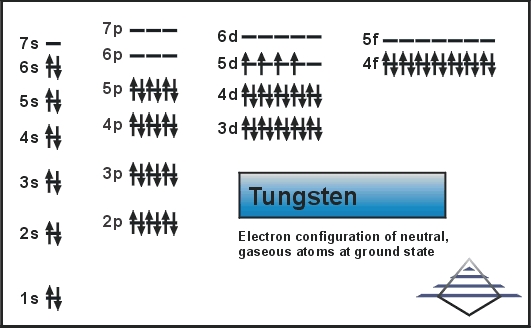
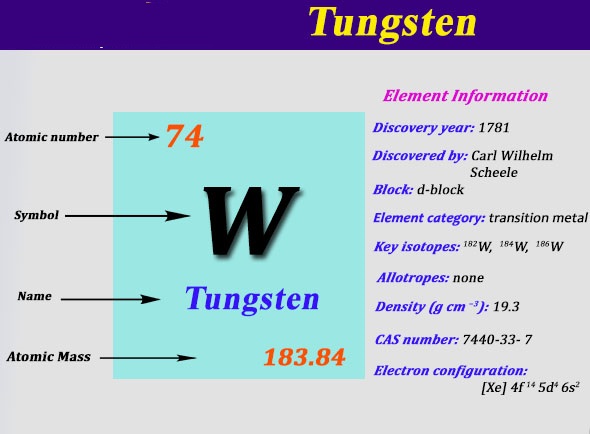
Electron Configuration For Tungsten: Tungsten is also known as Wolfram. It is a chemical element which has a chemical symbol W. The atomic number of tungsten is 74. The name tungsten has come from the prior Swedish name for the tungstate mineral scheelite from tungsten ( Which means a “heavy stone”). It is a rare metal that is found naturally on Earth.
It is almost found exclusively combined with other chemical elements rather than alone. Tungsten was identified as a new element in the year 1781 and first recognized as a metal in the year 1783. Its important ores are scheelite and wolframite.
The free element (tungsten) is remarkable for its robustness, especially the fact that tungsten has the highest melting point as compared to all the elements discovered, which is at 3422 °C . Also, it has the highest boiling point of 5930 °C.
The density of Tungsten is 19.3 times that of water, which is comparable to that of gold and uranium and much higher (approx 1.7 times) than that of lead. Polycrystalline tungsten is the exclusively hard and brittle material (under some conditions, when it is uncombined), which makes it difficult to work. However, the single-crystalline pure tungsten is more ductile and it can be cut with a hard-steel hacksaw.
Today we are here to share all the information about the electron configuration of tungsten. If you are here for the same then you are at the right place. Please go through the full article below to get more information as it can be really very helpful to you for studying tungsten in a better way.
What is the Electron Configuration of Tungsten?
[Xe] 4f14 5d4 6s2 is the electron configuration of tungsten
How Many Valence Electrons Does Tungsten Have
Tungsten has 74 valence electron in its outer shell.
Tungsten Number of Valence Electrons
There are 74 valence electrons in the outer shell of the tungsten.