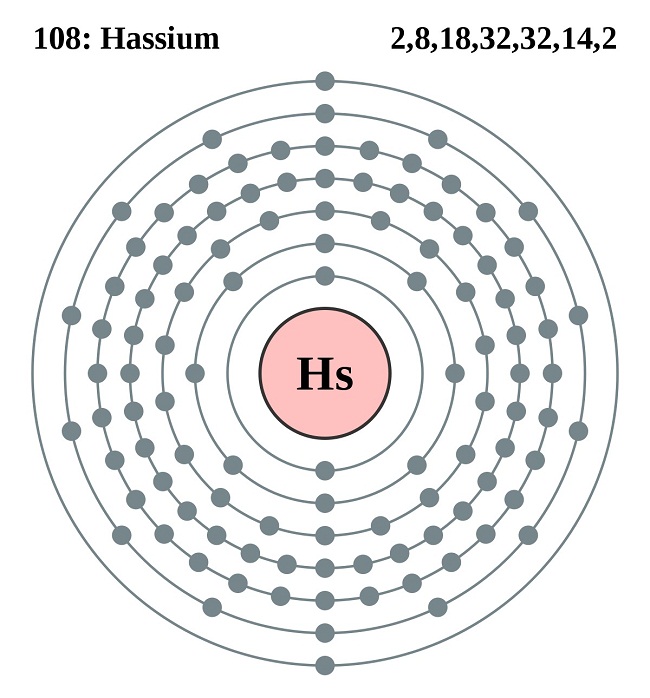
Hassium valence electrons study is available here for all the fanatics and chemistry scholars. Our such readers can here get to study and explore this element in its brief form. The article in periodic table contains the information on the basis of the introduction and the valency/valence electrons of the element. We, therefore, believe that the readers will be able to get ample information from the article for their basic understanding. Hassium is the chemical element that has the atomic number of 108 and the symbol of Hs.
The element is extremely radioactive in its nature to the extent of being lethal for human exposure. Almost all form of Hassium is laboratory-generated however there is the claim that element may also have its free form in the nature. This is also the super heavy element due to which it has only limited production capacity. The element is also quite famous for having the magic numbers of both electrons and protons. This feature of the element gives it a special place of usage in the nuclear domain.
How Many Valence Electrons Does Hassium Have?
Hassium has the 8 (Eight) valence electrons in the periodic table that are responsible to take part in the combining process. In nutshell, the valence electrons of Hassium are the number of electrons that it has in its outer shell. All the chemical elements have their own electrons numbers in the outer shell and we reckon them as the valence electrons.
Hassium Valence Electrons Dot Diagram
Well, for a better understanding of the Hassium valence electrons our readers can go with the lewis dot diagram. This is the specific diagram that is useful to visualize the valence electrons of any element. The diagram works with the dots that represent the numbers of valence electrons for the given element. So, if you are someone who tends to get in-depth for the understanding of Hassium valence electrons then this is the diagram for you. You can use it to study the chemical element in a thorough manner.
Valency of Hassium
If you wish to figure out the actual combining capacity of any element then you need to figure out its valency. As far as the Hassium is concerned it carries the valency of 7,4,3,2 respectively. It simply means that the element may gain or lose these numbers of electrons so as to get a stable electron configuration.