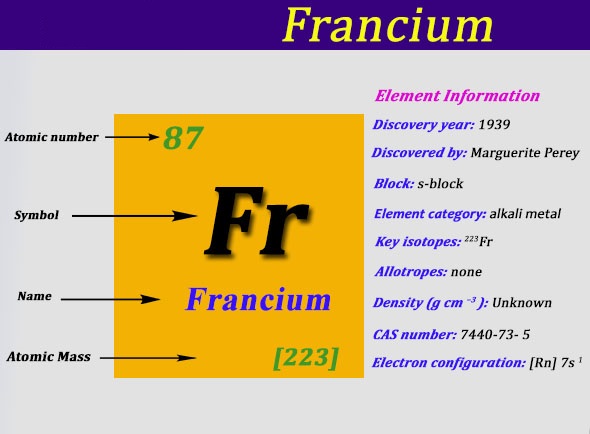
Electron Configuration For Francium: Francium is a chemical element that has a chemical symbol Fr. The atomic number of Francium is 87. It was earlier known as Eka-caesium. Francium is extremely radioactive. It is the most stable isotope, francium-223 ( it was originally called actinium K but after the natural decay chain it appears in) has a very short life of only 22 minutes.
Francium is the second-least electronegative element, after only caesium. After astatine, it is the second rarest naturally occurring element. The isotopes of francium quickly decay into radium, astatine, and radon. Since it is an alkali metal, it has only one valence electron. Francium has never been viewed in bulk. Because of the common appearance of the other elements in its column of a periodic table, it is pre-assumed that francium will appear as a very highly reactive metal if it is collected enough together to be seen as a bulk liquid or solid.
Getting such a sample is very improbable. It was discovered in France (from which the element takes its name) by Marguerite Perey in 1939. Francium was the last element that was first discovered in nature, rather than by synthesis. Outside the labs, it is extremely rare, with a trace of some amount found in thorium and uranium ores, where the isotope of francium-223 continuously forms and decays.
Today we are here to provide you with the information about the electron configuration of the Francium. It helps you to understand this element in a better way. if you are also here for the same then you are in the right place. Please go through the full post below.
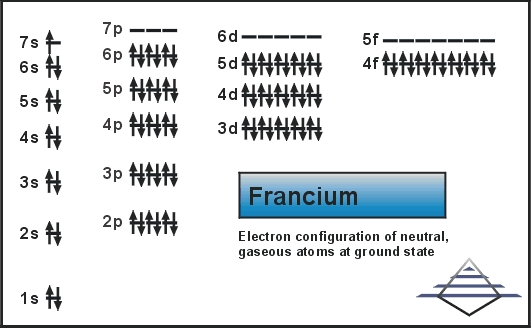
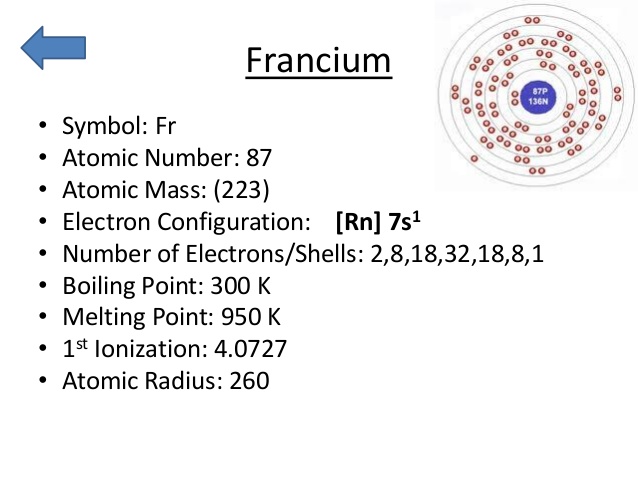
What is the Electron Configuration of Francium?
Rn 7s1 is the electron configuration of Francium.
How Many Valence Electrons Does Francium Have
Francium has only one valence electrons in its outer shell.
Francium Number of Valence Electrons
There is only valence electron in the outer shell of the Francium.
We hope you find this article useful.