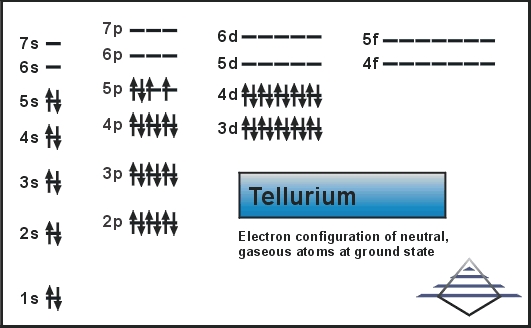
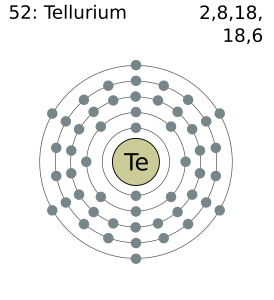
Electron Configuration For Tellurium: Tellurium is a chemical element that has a chemical symbol Te. The atomic number of tellurium is 52. It is mildly toxic, brittle, silver and rare metalloid. It is chemically related to sulfur and selenium. It is sometimes found in native form as elemental crystals.
Tellurium is more commonly found in the universe as a whole than it is found on Earth. It is found extremely rarely in the Earth’s crust, as compared to that of platinum. Tellurium is partly due to its high atomic number and also to the formation of volatile hydride that is caused it to get lost to space in form of gas during the hot nebular formation of the planet Earth.
Tellurium-bearing compounds were first discovered in a gold mine in Zlatna, Romania by Austrian mineralogist Franz-Joseph Müller von Reichenstein in 1782. However it was Martin Heinrich Klaproth who in 1798 named it as Tellurium; after the Latin word for “earth”, tell us.
Gold telluride minerals are the most notable and common natural gold compounds. Although Gold telluride is not a commercially a significant source of tellurium itself, that is usually extracted as a by-product of lead and copper production. The primary commercial use of tellurium is steel and copper alloys, where it helps to improve machinability.
Today we are here to share all the information about the electron configuration of Tellurium. If you are here for the same then you are in the right place. Please see the full post to get the more information.
What is The Electron Configuration of Tellurium?
[Kr] 4d10 5s2 5p4 is the electron configuration for tellurium
How Many Valence Electrons Does Tellurium Have
Tellurium has six valence electrons in its outer shell.
Tellurium Number of Valence Electrons
There are six valence electrons in the outer shell of the tellurium.
We hope you find all this information useful.