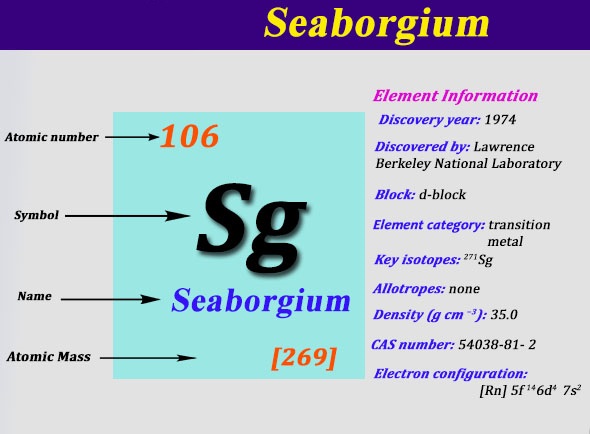
Electron Configuration For Seaborgium: Seaborgium is a synthetic chemical element which has a chemical symbol Sg. The atomic number of Seaborgium is 106. It got its name after the American nuclear chemist Glenn T. Seaborg. Since it is a synthetic element, it Is only created in a laboratory but is not found in nature. It is also radioactive and the most stable known isotope, 269Sg.
It has a half-life of approx 3.1 minutes. It is an ad-block transactinide element, in the periodic table of the elements. It is the member of the 7th period which belongs to the group 6 elements as it is the fourth member of the 6d series of transition metals. Chemical experiments have confirmed that it behaves as the heavier homologue than tungsten in group 6.
The chemical characteristics of seaborgium are shown only partly, but they are compared well with the chemistry of the other elements of group 6. A few atoms of seaborgium were produced in laboratories in the Soviet Union and in the United States in 1974.
The priority of its discovery and after that the naming of the element was disputed between American scientists and Soviet, and it was not until 1997 that IUPAC (International Union of Pure and Applied Chemistry) confirmed seaborgium as the official name for the element. It is one of only two elements that are named after a living person at the time of naming, the other on is oganesson, element 118.
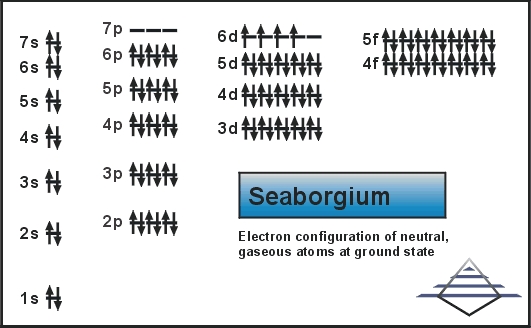
What is the Electron Configuration of Seaborgium?
[Rn] 5f14 6d4 7s2
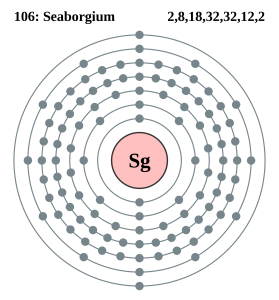
How Many Valence Electrons Does Seaborgium Have
It has 14 valence electrons in its outer shell.
Seaborgium Number of Valence Electrons
There are 14 valence electrons in the outer shell of the Seaborgium