Explore the Caesium electron configuration here and build a solid understanding of the chemical properties of the element. The article contains a brief discussion on the electron configuration and other significant properties of this element.
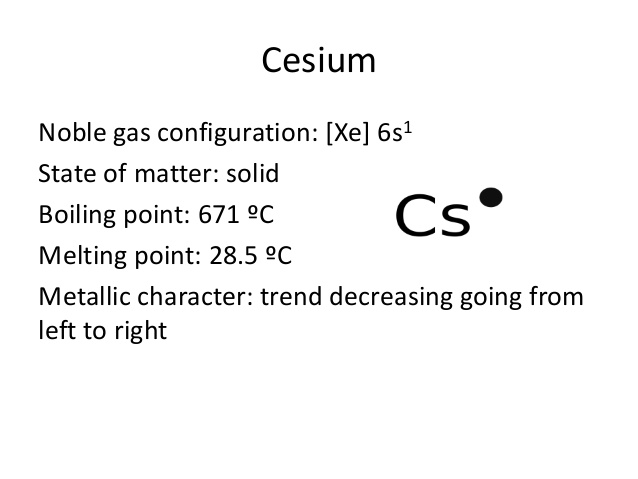
Caesium is the chemical element in the subject of chemistry that also holds its place in the periodic table. The element has the atomic number 55 and the representative symbol of Cs. The element belongs to the category of Alkali metals and has a physical structure like the soft silvery golden metal. Caesium is closely associated with the Rubidium and Potassium in the context of chemical properties.
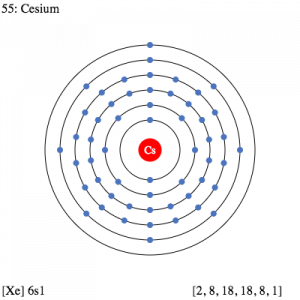
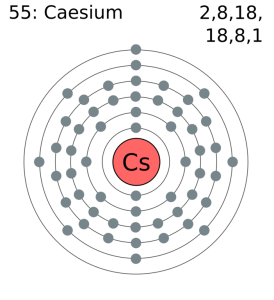
Caesium Electron Configuration
Caesium has the occurrence form from the mining of pollucite and also has some other minor forms of occurrence. The chemical element was first discovered by German scientists in the year of 1860. It’s quite an old chemical variant in itself that is useful for a number of practical domains.
Furthermore, Caesium is one of those chemical elements that have highly reactive properties. For the same reason, the element can easily react even with the water. It has mostly the liquid form even at the regular room temperature of around 28 degrees. So, you can basically expect the solid form of this chemical element at a temperature of below 15 or even lesser degrees temperature.
How many valence electrons does Caesium have?
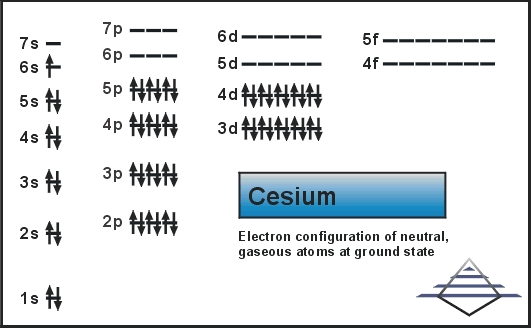
The electron configuration knowledge of Caesium is extremely significant for all the scholars of chemistry. It’s because the electron configuration for the chemical element depicts all the other important properties of the element. So, Caesium electron configuration is basically 1s22s22p63s23p64s23d104p65s24d105p66s1 in its descriptive long written form.
The electron configuration of Caesium is nothing but the representation of the electrons of the element to its orbitals. This electron distribution equation is what becomes the electron configuration of the element. This configuration of the electron for the chemical element is useful in the further research of the chemical element. For instance, the electron configuration helps in figuring out all the other relevant chemical properties of Caesium or any other element.
Electron Configuration for Cs
Well, Caesium is a highly practical chemical element that has a number of significant applications. The major use of the Caesium goes to the oil industry where the chemical element is used in oil extraction related activities. It works as the drilling fluid for the same oil extraction purpose. So, for the same reason, Caesium remains the highly demanded product in the oil industry.
Furthermore, Caesium has other numbers of usages such as in the atomic clocks or in the production of thermionic generators. This generator does the particular job of converting the heat energy to electricity. So, Caesium has a huge role to play in energy production and conversion.
So, this is all about the Caesium chemical element to understand the electron configuration and other properties of the element. We believe the article would prove to be beneficial in the study of same.