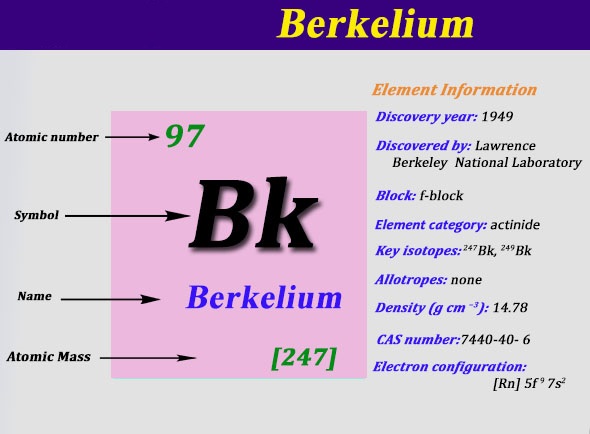
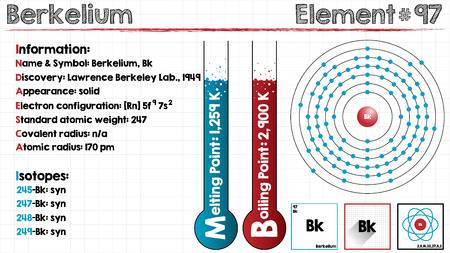
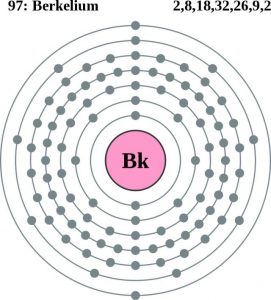
Electron Configuration For Berkelium: Berkelium is a radioactive transuranic chemical element that has a chemical symbol Bk. The atomic number of Berkelium is 97. It is a member of the transuranium and actinide element series.
Berkelium is named after the city of Berkeley, California, which is the location of the Lawrence Berkeley National Laboratory where in December 1949 it was discovered. It was the fifth transuranium element discovered after plutonium, neptunium, americium and curium.
The main isotope of berkelium is 249Bk. It is synthesized in very fewer quantities in high-flux dedicated nuclear reactors, mainly in Tennessee at the Oak Ridge National Laboratory, USA, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The production of its second-most important isotope 247Bk involves the rare isotope 244Cm irradiation with high-energy alpha particles.
Today we are here to share the information about the electron configuration of the Berkelium and also we will tell you about the valence electrons present in its outer shell. If you are also here to get the same information about the element then you are in the right place. Please go through the full post below.
What is the Electron Configuration of Berkelium
Rn 5f9 7s2 is the electron configuration of the Berkelium.
How Many Valence Electrons Does Berkelium Have
Berkelium has four valence electrons in its outer shell.
Berkelium Number of Valence Electrons
There are four valence electrons in the outer shell of the Berkelium.