Electron Configuration For Actinium: Electron Configuration is basically the distribution of the electron of any chemical element this distribution is made in the molecular or the atomic orbital. Electron Configuration has always been the most accurate source of determining the chemical reaction of any element.
Today in this article we are going to discuss the electron configuration of the Actinium.
Electron Configuration For Actinium
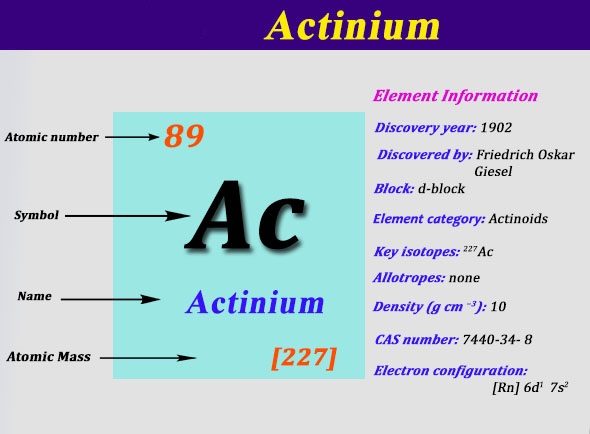
Actinium which is a chemical element is one of the rarest chemical elements on the earth. This chemical element was discovered long back in the year 1902 and it has the soft silver body structure.
Electron Configuration for Actinium helped the scientists and the chemist to understand the chemical reaction of the element so that it can be used in the appropriate usages.
You can get the complete information about this chemical element in the periodic table along with the electron configuration of Actinium.
What is the Electron Configuration of the Actinium
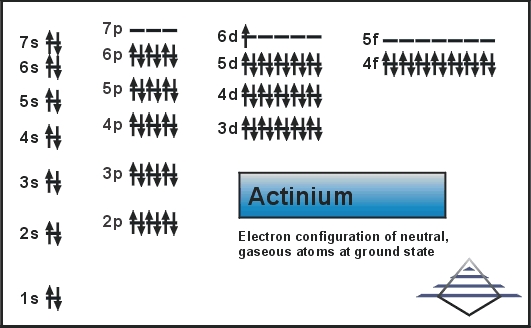
Actinium which is a chemical element with an atomic number of 89 and is symbolized by the Ac. The electron configuration of the Actinium is written as 6d1 7s2. The explanation behind this electron configuration of the Actinium is quite complicated for the majority of the students.
In this electron configuration of the Actinium, it is assumed that the energy increases from the left to the right and this is the reason, that why we write it as 6d1 which is later followed by the7s2 as the 7s has the higher energy than the 6d.
It is called the oversimplification concept by which the s sublevel is filled before the d sublevel.
In the electron configuration of any chemical element, the energy of the sub-levels can’t be kept as fixed, as it keeps on varying in accordance with the atomic number of the chemical element.
How Many Valence Electron Does Actinium Have
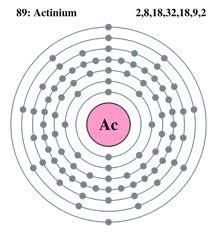
Actinium holds the 2 Valence of electrons within it and you can easily cross-check the number of Valence for any chemical element by referring the group number of the element in the periodic chemical element table.
Further, the Actinium has 89 protons and the 138 neutrons with the 227.0 units of the atomic mass.
Actinium Number of Electron Valence
Actinium is having the valence of 2 electrons along with the 138 neutrons and the 89 protons. Actinium is considered to be a very rare chemical element on the earth which is found solely in the ores of Uranium.
The element is highly radioactive which generates the blue light and behaves in the same way with IANTHANUM. This chemical element starts melting at the temperature of 1050 degrees.