What are the Understanding of Electron Cloud Model? Electronic Cloud Model is an atomic model proposed by Erwin Schrödinger and Werner Heisenberg in 1926. Through this model, they wanted to explain the probable position of an electron in an atom. According to this model, electrons do not revolve around the nucleus in straight elliptical paths, as proposed by the earlier models. On the contrary, they are found in clouds at specific places near the nucleus of an atom, or even inside it.
What are the Understanding of Electron Cloud Model
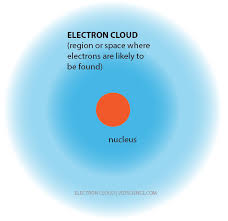
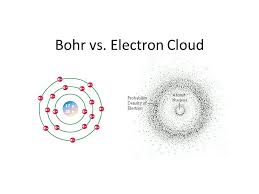
The electron cloud model is a representation of the behavior and distribution of electrons within an atom. According to this model, electrons do not follow fixed paths or orbits around the nucleus, as proposed in the Bohr model. Instead, electrons are described as existing in regions of probability called electron clouds or electron orbitals. These electron clouds are three-dimensional regions around the nucleus where electrons are likely to found. The model takes into account the dual nature of electrons, behaving as both particles and waves, and the uncertainty principle proposed by Werner Heisenberg.
In the electron cloud model, the nucleus is at the center, surrounded by different energy levels or shells. Each shell contains one or more subshells, and each subshell contains one or more orbitals. Electrons occupy these orbitals in pairs with opposite spins, following the Pauli exclusion principle. The model provides a more accurate description of an atom’s electron distribution and allows for more complex and realistic chemical bonding patterns.
Furthermore, the electron cloud model is fundamental in explaining the periodicity and properties of elements in the periodic table. It enables scientists to predict and understand the behavior of atoms in various chemical reactions, as well as in the formation of molecules and compounds. The model is an essential tool for modern chemistry and plays a crucial role in advancing our understanding of the atomic and molecular world.
Electron Cloud Model of the Atom
The electron cloud model of the atom, also known as the quantum mechanical model or the wave-mechanical model, is a depiction of how electrons behave within an atom. It emerged as a significant advancement over earlier models like the Bohr model. Proposed in the 1920s and 1930s by physicists such as Erwin Schrödinger, Werner Heisenberg, and others, the electron cloud model is based on quantum mechanics, which describes the behavior of particles on a subatomic scale.
In this model, electrons considered to have both particle-like and wave-like properties. Instead of following specific paths around the nucleus, electrons described by wave functions that represent the probability of finding an electron in a particular region around the nucleus. These regions called orbitals or electron clouds, and they come in various shapes and sizes, corresponding to different energy levels and subshells.
The electron cloud model allows us to understand the organization of electrons within an atom, the filling of electron shells and subshells, and the patterns of chemical bonding. It has led to a more comprehensive and accurate understanding of atomic structure and has paved the way for advancements in various fields, including chemistry, materials science, and electronics.
When Was the Electron Cloud Model Discovered?
The development of the electron cloud model emerged during the early 20th century, as scientists delved into understanding the behavior of electrons and the structure of atoms. The initial breakthrough came with the formulation of quantum mechanics in the mid-1920s. Quantum mechanics, developed by physicists such as Werner Heisenberg, Erwin Schrödinger, Max Born, and others, provided a new framework for describing the behavior of subatomic particles, including electrons.
The electron cloud model, also referred to as the quantum mechanical model or wave-mechanical model, started to take shape in the late 1920s and early 1930s. In 1926, Schrödinger published his famous wave equation, which described the behavior of electrons as waves rather than particles in fixed orbits. At the same time, Heisenberg proposed the uncertainty principle, emphasizing that it is impossible to know both the position and momentum of an electron simultaneously.
The electron cloud model represented a departure from the classical models of atomic structure and provided a more accurate description of electron behavior. It gradually gained acceptance and became the prevailing model to describe the atom’s electronic structure by the mid-20th century. Since then, it has served as a fundamental pillar of modern physics and chemistry.
Who Created the Electron Cloud Model
The electron cloud model, also known as the quantum mechanical model or wave-mechanical model, was the result of collaborative efforts by several pioneering physicists during the early 20th century. The development of this model can attributed to notable figures such as Erwin Schrödinger, Werner Heisenberg, Max Born, and Louis de Broglie.
In 1926, Erwin Schrödinger formulated the Schrödinger equation, a fundamental equation of quantum mechanics that describes the behavior of particles, including electrons, as wave-like entities. His work laid the foundation for the wave function theory, where electrons described by wave functions representing probabilities of their positions in space.
At the same time, Werner Heisenberg proposed the uncertainty principle in 1927, highlighting the limitations in simultaneously knowing the position and momentum of a particle. This principle added a significant aspect to the understanding of electron behavior and was an essential contribution to the electron cloud model.
Max Born’s interpretation of the wave function as a probability density in 1926 also played a crucial role in shaping the electron cloud model. This interpretation provided a mathematical understanding of the probabilistic nature of quantum mechanics.
The collective contributions of these physicists, along with the earlier work of Louis de Broglie, Niels Bohr, and others, led to the establishment of the electron cloud model, which revolutionized our understanding of atomic structure and set the stage for the development of modern physics.
What is the Charge of the Electron Cloud?
The electron cloud, by definition, refers to the region around the nucleus of an atom where electrons likely to found. Electrons elementary particles that carry a negative electric charge. Each individual electron has a charge of approximately -1.602 x 10^-19 coulombs. The negative charge of electrons is balanced by the positive charge of protons within the atomic nucleus, resulting in an overall neutral charge for the atom.
In the electron cloud model, electrons distributed in different energy levels, or shells, around the nucleus. These shells further divided into subshells and orbitals. Each orbital can hold a maximum of two electrons with opposite spins, following the Pauli exclusion principle.
The distribution of electrons within the electron cloud is crucial in determining an atom’s chemical properties and reactivity. The arrangement of electrons in the outermost shell, known as the valence electrons, plays a significant role in the formation of chemical bonds and interactions with other atoms.
The charge of the electron cloud is integral to the overall stability and behavior of atoms. Understanding the electron cloud’s charge and its distribution allows scientists to comprehend the complex chemical reactions that occur in nature and essential for the functioning of biological systems, materials, and the technologies we rely on in our daily lives.